How Is Specific Gravity Related To Density
Muz Play
Mar 19, 2025 · 6 min read
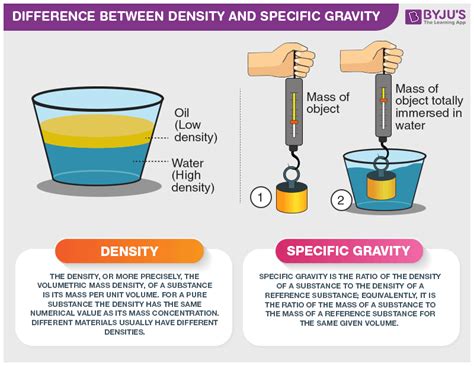
Table of Contents
How is Specific Gravity Related to Density? A Deep Dive
Specific gravity and density are two closely related concepts frequently used in various scientific and engineering fields. While often used interchangeably in casual conversation, they are distinct measurements with a crucial mathematical relationship. Understanding this relationship is essential for accurately interpreting data and solving problems involving fluid mechanics, material science, and other related disciplines. This article will delve into the specifics of density and specific gravity, clarifying their definitions, explaining their relationship, exploring their applications, and highlighting the differences between them.
Understanding Density
Density is a fundamental physical property of matter, defining the mass of a substance per unit volume. It essentially tells us how much "stuff" is packed into a given space. A higher density means more mass is concentrated within a smaller volume. The standard unit for density is kilograms per cubic meter (kg/m³), although other units like grams per cubic centimeter (g/cm³) are also commonly used. The formula for density is:
Density (ρ) = Mass (m) / Volume (V)
For example, a substance with a density of 1 g/cm³ means that one cubic centimeter of that substance has a mass of one gram. Different materials have vastly different densities. Lead, for instance, is significantly denser than wood, meaning a given volume of lead will have a much greater mass. This difference in density stems from the arrangement of atoms within the material and the mass of the atoms themselves.
Factors Affecting Density
Several factors can influence a substance's density:
-
Temperature: Generally, density decreases as temperature increases. This is because increased thermal energy causes molecules to move farther apart, expanding the volume while the mass remains constant. However, there are exceptions to this rule, particularly with water near its freezing point.
-
Pressure: Increasing pressure usually increases density because it compresses the substance, reducing its volume. This effect is more pronounced in gases than in liquids or solids.
-
Composition: The chemical composition of a material significantly influences its density. Different elements and molecules have different atomic masses and packing arrangements, leading to variations in density.
-
Phase: The phase of a substance (solid, liquid, or gas) drastically affects its density. Generally, solids are the densest, followed by liquids, and then gases. This is because the molecules in solids are closely packed together, while those in gases are widely dispersed.
Understanding Specific Gravity
Specific gravity (SG), also known as relative density, is a dimensionless quantity that compares the density of a substance to the density of a reference substance. The reference substance is typically water at 4°C (39.2°F), which has a density of approximately 1000 kg/m³ or 1 g/cm³.
Specific Gravity (SG) = Density of Substance / Density of Reference Substance
Because specific gravity is a ratio of two densities, it's a dimensionless number. This means it has no units, making it easier to interpret and compare across different substances. For example, a substance with a specific gravity of 2 is twice as dense as water, and a substance with a specific gravity of 0.5 is half as dense as water.
Why Use Specific Gravity?
Specific gravity offers several advantages over simply using density:
-
Simplicity: Specific gravity provides a readily understandable comparison to a well-known reference, eliminating the need for complex unit conversions.
-
Ease of measurement: Specific gravity can be measured using relatively simple instruments like hydrometers, making it a practical tool for field measurements.
-
Temperature independence (to an extent): While temperature affects both the substance's density and the reference substance's density, the effect often cancels out partially, providing a more stable measurement compared to density alone, especially when dealing with similar temperature ranges.
The Relationship Between Density and Specific Gravity
The relationship between density and specific gravity is direct and straightforward. Specific gravity is calculated from the density of a substance and the density of a reference substance (usually water). Therefore, if you know the density of a substance, you can easily calculate its specific gravity, and vice-versa.
The formula can be rearranged to solve for density if the specific gravity is known:
Density of Substance = Specific Gravity × Density of Reference Substance
For example, if a substance has a specific gravity of 1.5 (relative to water), and the density of water is 1 g/cm³, then the density of the substance is 1.5 g/cm³.
Applications of Density and Specific Gravity
Both density and specific gravity find extensive applications across numerous fields:
1. Fluid Mechanics:
-
Determining buoyancy: Whether an object floats or sinks in a fluid depends on the object's density relative to the fluid's density. This principle is crucial in naval architecture, submarine design, and other related areas. Specific gravity is frequently used in this context.
-
Fluid flow analysis: Density is a critical parameter in equations governing fluid flow, pressure, and other properties.
2. Material Science and Engineering:
-
Material identification: Density and specific gravity are useful characteristics for identifying unknown materials or verifying the purity of known materials.
-
Material selection: Density considerations are vital in engineering design, as they influence the weight and structural integrity of components.
3. Chemical Engineering:
-
Concentration measurements: Specific gravity is often used to determine the concentration of solutions, particularly in industrial processes.
-
Process control: Monitoring density or specific gravity helps in controlling the quality and consistency of various chemical processes.
4. Medical Applications:
-
Urine analysis: The specific gravity of urine is an indicator of hydration levels and kidney function.
-
Blood analysis: Density and related measurements provide insights into blood composition and health status.
5. Geology and Hydrology:
-
Rock and mineral identification: Density is a key property used in identifying different types of rocks and minerals.
-
Groundwater studies: The density of groundwater can provide clues about its composition and potential contamination.
Differences Between Density and Specific Gravity
While interconnected, density and specific gravity have key differences:
| Feature | Density | Specific Gravity |
|---|---|---|
| Definition | Mass per unit volume | Ratio of substance density to reference density |
| Units | kg/m³, g/cm³, lb/ft³ | Dimensionless |
| Measurement | Requires mass and volume measurements | Often measured directly using a hydrometer |
| Reference | No specific reference | Usually compared to water at 4°C |
Conclusion
Density and specific gravity are essential concepts in various scientific and engineering disciplines. While specific gravity is derived from density, it offers advantages in terms of simplicity and ease of measurement. Understanding the relationship between these two parameters and their individual applications is vital for solving problems and making informed decisions in diverse fields. This detailed explanation, enriched with examples and applications, aims to provide a comprehensive understanding of these critical concepts, allowing for more effective problem-solving and a deeper appreciation for the physical properties of matter.
Latest Posts
Latest Posts
-
Is Alcohol A Acid And A Base Bronsted
Mar 19, 2025
-
Do Acids Gain Or Lose Hydrogen Ions
Mar 19, 2025
-
Lewis Diagram For A Ion With A Total Of Electrons
Mar 19, 2025
-
A The Symbol For Sample Standard Deviation Is
Mar 19, 2025
-
What Are The Columns In The Periodic Table Called
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about How Is Specific Gravity Related To Density . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
