Each Element In An Element Family Shares The Same
Muz Play
Mar 16, 2025 · 6 min read
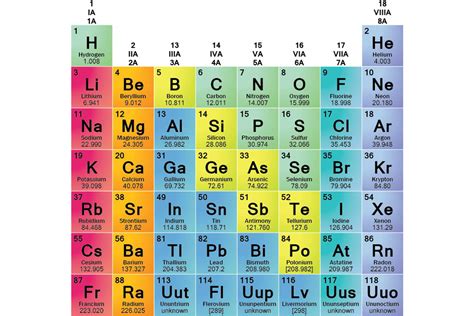
Table of Contents
Each Element in an Element Family Shares the Same: Exploring Periodic Trends
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and resulting properties. Elements are grouped into families, or groups, in columns, and these families exhibit striking similarities. The fundamental reason behind this shared behavior lies in the fact that each element in an element family shares the same number of valence electrons. This seemingly simple statement underpins a wealth of predictable chemical and physical properties. Let's delve deeper into this crucial concept and explore the implications across various element families.
Valence Electrons: The Key to Family Resemblance
The outermost shell of an atom, known as the valence shell, houses the valence electrons. These electrons are the primary participants in chemical bonding, dictating how an atom will interact with other atoms. Elements within the same family possess the same number of valence electrons. This shared characteristic directly influences their reactivity, bonding patterns, and overall chemical behavior.
Why Valence Electrons Matter:
-
Chemical Bonding: Valence electrons are responsible for the formation of chemical bonds. Atoms tend to react in ways that achieve a stable electron configuration, often by gaining, losing, or sharing valence electrons. Elements with similar numbers of valence electrons will exhibit similar bonding behaviors.
-
Reactivity: The number of valence electrons strongly influences an element's reactivity. Elements with nearly full valence shells (like halogens) are highly reactive because they readily gain electrons to complete their shells. Conversely, elements with only a few valence electrons (like alkali metals) are also highly reactive, readily losing their valence electrons to achieve a stable configuration.
-
Oxidation States: The oxidation state of an element reflects its apparent charge in a compound. This charge is directly related to the number of valence electrons gained, lost, or shared during bonding. Elements within the same family typically exhibit similar oxidation states.
-
Physical Properties: While not as directly influenced as chemical properties, physical properties like melting point and boiling point can also show trends within a family due to the influence of valence electrons on interatomic forces.
Exploring Key Element Families: A Detailed Look
Let's examine some prominent element families and illustrate how their shared number of valence electrons manifests in their properties and behavior.
1. Alkali Metals (Group 1): The Lone Electron
Alkali metals, including lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr), all share a single valence electron. This characteristic explains their striking similarities:
-
High Reactivity: They readily lose their single valence electron to form a +1 ion, achieving a stable electron configuration like the noble gases. This explains their vigorous reactions with water and other substances.
-
Low Ionization Energy: Removing the lone valence electron requires relatively little energy.
-
Low Electronegativity: They have a low tendency to attract electrons.
-
Soft Metals: They are soft enough to be cut with a knife.
-
Low Melting and Boiling Points: Compared to other metals, they have relatively low melting and boiling points.
Note: The properties of Francium are less well-known due to its extreme radioactivity and short half-life.
2. Alkaline Earth Metals (Group 2): Two Valence Electrons
Alkaline earth metals such as beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra) possess two valence electrons. Their shared properties include:
-
Reactivity (though less than Alkali Metals): They tend to lose their two valence electrons to form +2 ions. Their reactivity increases down the group.
-
Higher Ionization Energy than Alkali Metals: Removing two electrons requires more energy than removing one.
-
Higher Melting and Boiling Points than Alkali Metals: The stronger metallic bonding due to two valence electrons contributes to higher melting and boiling points.
-
Relatively Hard Metals: They are harder than alkali metals.
Note: Beryllium shows some anomalous behavior due to its small atomic size.
3. Halogens (Group 17): One Electron Short
Halogens – fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At) – all have seven valence electrons. Their shared characteristics stem from their tendency to gain one electron:
-
High Reactivity: They readily gain one electron to form a -1 ion, achieving a stable octet configuration.
-
High Electronegativity: They strongly attract electrons.
-
High Electron Affinity: They release significant energy when they gain an electron.
-
Diatomic Molecules: They exist as diatomic molecules (e.g., F₂, Cl₂) in their elemental form.
-
Varied Physical States: Fluorine and chlorine are gases, bromine is a liquid, and iodine is a solid at room temperature. This illustrates the effect of increasing atomic size on physical state.
Note: Astatine is highly radioactive and its properties are less well-characterized.
4. Noble Gases (Group 18): The Inert Ones
Noble gases – helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn) – possess a full valence shell (except helium, which has a full shell with two electrons). This explains their exceptional inertness:
-
Very Low Reactivity: They have little tendency to gain, lose, or share electrons because their valence shells are already full.
-
High Ionization Energies: Removing an electron is extremely difficult.
-
High Electronegativity: They possess minimal electronegativity due to their stable electron configurations.
-
Monoatomic Gases: They exist as monatomic gases under normal conditions.
Note: While generally inert, heavier noble gases like xenon can participate in some chemical reactions under specific conditions.
5. Transition Metals: Variable Valence Electrons
Transition metals represent a more complex case. While they don't share the same exact number of valence electrons, they often exhibit multiple oxidation states due to the involvement of both the (n-1)d and ns electrons in bonding. This leads to a broader range of chemical behaviors compared to the main group elements.
Predicting Properties: The Power of Periodic Trends
The shared valence electron count within a family allows us to predict many properties. Several periodic trends are observable:
-
Atomic Radius: Atomic radius generally increases down a group due to the addition of electron shells.
-
Ionization Energy: Ionization energy generally decreases down a group due to the increasing distance between the valence electrons and the nucleus.
-
Electronegativity: Electronegativity generally decreases down a group as the valence electrons are further from the nucleus.
-
Metallic Character: Metallic character generally increases down a group.
Conclusion: A Unified Understanding
The consistent number of valence electrons within each element family provides a powerful framework for understanding the remarkable similarities in their chemical and physical properties. By recognizing this fundamental principle, we can predict the behavior of elements and understand the intricate relationships that govern the chemical world. This understanding forms the basis for numerous applications in chemistry, materials science, and beyond, emphasizing the crucial role of the periodic table in our comprehension of matter. The consistent behavior within families offers a powerful tool for predicting properties and designing new materials with specific characteristics. Further research continues to refine our understanding of these trends and the subtle nuances within each family, pushing the boundaries of chemical knowledge.
Latest Posts
Latest Posts
-
Factors That Influence The Elasticity Of Supply
Mar 17, 2025
-
Describe How The Atoms In A Compound Are Held Together
Mar 17, 2025
-
How Is Absorbance Linked To Rate Of Reaction
Mar 17, 2025
-
What Happens To Electrons In An Ionic Bond
Mar 17, 2025
-
Are Antiparalell Beta Sheets Mrore Stable
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Each Element In An Element Family Shares The Same . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
