Elements In The Same Group Have
Muz Play
Mar 18, 2025 · 6 min read
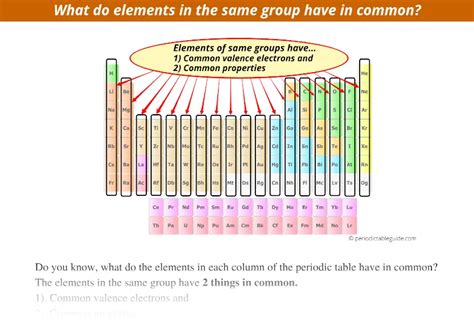
Table of Contents
Elements in the Same Group Have Similar Properties: A Deep Dive into Periodic Trends
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and resulting properties. Understanding the relationships between elements is crucial for predicting their behavior and applications. A key observation is that elements in the same group (vertical column) have similar properties. This similarity isn't coincidental; it stems from the identical number of valence electrons, the outermost electrons responsible for chemical bonding. This article will explore the reasons behind this similarity, examining various properties and delving into exceptions to the rule.
Valence Electrons: The Key to Group Similarity
The defining characteristic of elements within the same group is their identical number of valence electrons. These electrons are located in the outermost electron shell and are the primary participants in chemical bonding. For example, all elements in Group 1 (alkali metals) have one valence electron, while Group 18 (noble gases) possess eight (except for helium, which has two). This consistent number of valence electrons directly impacts several key properties:
1. Chemical Reactivity:
The number of valence electrons dictates an element's reactivity. Elements strive to achieve a stable electron configuration, often resembling that of the noble gases (octet rule). Elements with nearly full valence shells (like halogens in Group 17) readily gain electrons to complete their octet, exhibiting high reactivity. Conversely, elements with only a few valence electrons (like alkali metals) readily lose electrons to achieve a stable configuration, also exhibiting high reactivity. Elements with full valence shells (noble gases) are largely unreactive due to their stable configuration. This trend is clearly visible across groups: the reactivity of alkali metals increases down the group (easier to lose the outer electron as atomic size increases), while the reactivity of halogens decreases down the group (increasing atomic size makes it harder to gain an electron).
2. Oxidation States:
The common oxidation states of elements within a group are closely related to their number of valence electrons. Elements tend to lose or gain electrons to achieve a stable electron configuration, resulting in specific oxidation states. For example, alkali metals consistently exhibit a +1 oxidation state because they readily lose their single valence electron. Halogens commonly show a -1 oxidation state due to their tendency to gain one electron to complete their octet. Transition metals, however, can exhibit multiple oxidation states because of the involvement of both (n-1)d and ns electrons in bonding. While not as predictably consistent as main group elements, trends in oxidation states still exist within transition metal groups.
3. Ionization Energy:
Ionization energy is the energy required to remove an electron from a neutral atom. Within a group, ionization energy generally decreases as you move down. This is because the increasing atomic radius results in a weaker attraction between the nucleus and the outermost electron, making it easier to remove. The greater the distance between the nucleus and the valence electrons, the less tightly bound they are. This trend is consistent across all groups, although the magnitude of the decrease may vary depending on the electronic configuration and shielding effects.
4. Electronegativity:
Electronegativity measures an atom's ability to attract electrons in a chemical bond. Generally, electronegativity decreases down a group. This is primarily due to the increasing atomic radius; the nucleus's pull on bonding electrons weakens as the distance increases. However, there are exceptions to this trend, particularly in some transition metal groups where the effect of increased nuclear charge and d-electron shielding can be complex.
5. Atomic Radius:
Atomic radius, the distance from the nucleus to the outermost electron, increases down a group. As you descend a group, additional electron shells are added, increasing the overall size of the atom. This increase in size directly influences several other properties discussed above, including ionization energy and electronegativity. This consistent expansion of atomic size as you move down a group is a fundamental periodic trend.
6. Melting and Boiling Points:
Melting and boiling points are influenced by interatomic forces. The strength of these forces varies significantly across and within groups, often leading to less consistent trends than other properties. However, general trends can sometimes be observed. For instance, in Group 1, melting and boiling points tend to decrease down the group, primarily because of weakening metallic bonding with increasing atomic size and electron shielding. In Group 17 (halogens), the trend is less straightforward due to the increasing strength of van der Waals forces with increasing molecular size.
Exceptions and Nuances: Why the Rules Aren't Always Absolute
While the general principle that elements in the same group exhibit similar properties holds true, there are exceptions and nuances to consider. These deviations often stem from:
1. Relativistic Effects:
In heavier elements, particularly those at the bottom of groups, relativistic effects become significant. The increased velocity of inner electrons leads to a contraction of the s and p orbitals, impacting their shielding effect and altering properties like ionization energy and atomic radius. This is particularly noticeable in groups with heavy elements like gold and mercury.
2. Anomalous Behavior of the First Element:
The first element in a group often shows distinct differences from its heavier congeners. This is because the first element has a smaller atomic size, higher ionization energy, and higher electronegativity. The lack of inner electron shells leads to a more significant influence of the nucleus on the valence electrons, deviating from the trends observed in heavier elements. Lithium in Group 1 and Boron in Group 13 are prime examples of such anomalous behavior.
3. Electronic Configuration Variations:
While the number of valence electrons is crucial, variations in electronic configurations can affect properties. For instance, the transition metals exhibit variable oxidation states due to the participation of both (n-1)d and ns electrons in bonding. This leads to less predictable trends in properties compared to the main group elements.
4. Intermolecular Forces:
The strength of intermolecular forces can significantly influence melting and boiling points, even within the same group. Factors like molecular polarity and size play crucial roles. This can lead to irregular trends in these properties, as observed in Group 17 (halogens).
5. d and f Block Elements:
The transition metals (d-block) and inner transition metals (f-block) exhibit less predictable trends compared to the main group elements. The complex interplay of d and f electrons complicates the analysis of their properties, leading to variations in trends within the group.
Conclusion: A Powerful Predictive Tool
Despite the exceptions, the principle that elements within the same group share similar properties remains a powerful tool for predicting chemical behavior. The consistent number of valence electrons dictates many crucial aspects of an element's reactivity, bonding characteristics, and other fundamental properties. By understanding the underlying reasons for these similarities and appreciating the exceptions, chemists can leverage the periodic table to predict the behavior of elements and design new materials with tailored properties. While the periodic table offers a broad generalization, careful consideration of the exceptions and nuances is crucial for a complete understanding. Further investigation into the specific electron configurations and the influences of various factors helps refine predictions and enhance our understanding of chemical reactivity and the behavior of elements. This comprehensive understanding of periodic trends and their variations underscores the power and utility of the periodic table as a cornerstone of chemical knowledge and predictive capabilities.
Latest Posts
Latest Posts
-
Is S More Electronegative Than O
Mar 18, 2025
-
How Many Valence Electrons Are In Sulfur
Mar 18, 2025
-
What Is The Difference Between Volume Measurements And Capacities
Mar 18, 2025
-
In A Polar Covalent Bond Electrons Are Shared
Mar 18, 2025
-
Why Does Standard Deviation Decrease With Sample Size
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Elements In The Same Group Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
