Energy Stored In Chemical Bonds Is Called
Muz Play
Mar 17, 2025 · 7 min read
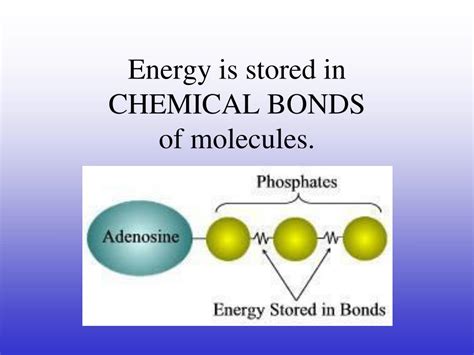
Table of Contents
Energy Stored in Chemical Bonds is Called Chemical Energy: A Deep Dive
Energy is the capacity to do work, and it exists in many forms. One crucial form is chemical energy, the potential energy stored within the chemical bonds of molecules. This energy is released or absorbed during chemical reactions, driving processes vital to life and technology. This comprehensive article delves into the nature of chemical energy, its origins, its role in various processes, and its practical applications. We will explore the factors influencing the strength of chemical bonds and the consequent energy storage, along with discussing the different types of chemical bonds and their energy content.
Understanding Chemical Bonds and Their Energy
Chemical bonds are the forces that hold atoms together in molecules. These bonds arise from the interactions between electrons in the outermost shells of atoms, known as valence electrons. The formation of a chemical bond involves a decrease in the overall energy of the system. This energy decrease represents the energy released when the bond is formed and is stored as potential energy within the bond itself. Conversely, breaking a chemical bond requires energy input, reflecting the energy stored within the bond.
Types of Chemical Bonds and Their Energy Content
Several types of chemical bonds exist, each with different strengths and corresponding energy storage capacities:
-
Covalent Bonds: These bonds involve the sharing of electrons between atoms. The strength of a covalent bond depends on factors such as the electronegativity of the atoms involved (their ability to attract electrons) and the number of electron pairs shared (single, double, or triple bonds). Generally, stronger covalent bonds store more energy. Examples include the C-H bond in hydrocarbons and the O=O bond in oxygen molecules.
-
Ionic Bonds: These bonds result from the electrostatic attraction between oppositely charged ions. One atom loses electrons to become a positively charged cation, while another atom gains electrons to become a negatively charged anion. The strength of an ionic bond depends on the charges of the ions and the distance between them. Ionic bonds are typically stronger than weaker covalent bonds, thus storing significant energy. Sodium chloride (NaCl), or common table salt, exemplifies an ionic bond.
-
Metallic Bonds: These bonds occur in metals, where valence electrons are delocalized and shared among many atoms. This creates a "sea" of electrons that holds the metal atoms together. The strength of a metallic bond depends on the number of valence electrons and the size of the atoms. The strength of metallic bonds contributes significantly to the properties of metals like malleability and conductivity.
-
Hydrogen Bonds: While weaker than covalent, ionic, or metallic bonds, hydrogen bonds are crucial in biological systems. They involve the attraction between a hydrogen atom bonded to a highly electronegative atom (like oxygen or nitrogen) and another electronegative atom. Although individually weak, numerous hydrogen bonds collectively contribute to the stability of many biomolecules like proteins and DNA. The energy stored within them is crucial for biological functions.
Factors Affecting Energy Storage in Chemical Bonds
Several factors influence the amount of energy stored within chemical bonds:
-
Bond Length: Shorter bond lengths generally correspond to stronger bonds and higher energy storage. This is because the closer the atoms are, the stronger the electrostatic attraction between them.
-
Bond Order: The number of electron pairs shared between atoms (single, double, or triple bond) affects bond strength. Higher bond orders mean stronger bonds and higher energy storage. A triple bond, for instance, is stronger than a double bond, which is stronger than a single bond.
-
Electronegativity: The difference in electronegativity between atoms involved in a bond affects its polarity and strength. A larger electronegativity difference leads to a more polar bond, which can be stronger or weaker depending on the specific atoms involved. Highly polar bonds often involve significant energy storage.
-
Resonance: In some molecules, electrons can be delocalized over several atoms, leading to resonance structures. This delocalization strengthens the bonds and increases energy storage. Benzene, with its delocalized pi electrons, is a classic example.
-
Steric Effects: The spatial arrangement of atoms and groups around a bond can influence its strength and stability. Steric hindrance, where bulky groups hinder bond formation or stability, can reduce energy storage.
Chemical Energy and its Role in Various Processes
Chemical energy plays a fundamental role in a wide array of processes:
1. Biological Systems:
-
Metabolism: Living organisms use chemical energy stored in food molecules (carbohydrates, fats, proteins) through a series of metabolic reactions. These reactions break down complex molecules, releasing energy that powers cellular processes. This energy is stored temporarily as ATP (adenosine triphosphate), the "energy currency" of cells.
-
Photosynthesis: Plants and other photosynthetic organisms use light energy to convert carbon dioxide and water into glucose, storing energy in the chemical bonds of glucose molecules. This process is the foundation of most food chains.
-
Cellular Respiration: The process of cellular respiration releases the chemical energy stored in glucose through a series of controlled reactions, providing energy for cellular functions.
2. Combustion:
Combustion is a rapid chemical reaction that releases a large amount of energy in the form of heat and light. This process involves the oxidation of a fuel (like hydrocarbons) by an oxidant (like oxygen), breaking chemical bonds in the fuel and forming new, lower-energy bonds in the products (carbon dioxide and water). This energy release is harnessed in power plants and internal combustion engines.
3. Batteries:
Batteries store chemical energy and convert it into electrical energy through redox reactions (reduction-oxidation). These reactions involve the transfer of electrons between different chemical species. The stored chemical energy drives the flow of electrons, producing an electric current.
4. Explosives:
Explosives store vast amounts of chemical energy that is rapidly released during detonation. The rapid expansion of gases produced during the reaction causes a powerful explosion. The chemical bonds in explosives are designed to be highly unstable, allowing for rapid and energetic release.
5. Industrial Processes:
Many industrial processes rely on chemical reactions that either release or absorb chemical energy. Examples include the production of ammonia (Haber-Bosch process), the cracking of petroleum, and the synthesis of polymers. Understanding and controlling the energy changes in these processes are crucial for efficiency and safety.
Measuring Chemical Energy: Enthalpy and Bond Energies
The energy change in a chemical reaction is quantified using thermodynamic concepts:
-
Enthalpy (H): Enthalpy is a measure of the total heat content of a system at constant pressure. The change in enthalpy (ΔH) during a reaction indicates whether the reaction is exothermic (releases heat, ΔH < 0) or endothermic (absorbs heat, ΔH > 0).
-
Bond Energy: Bond energy is the average amount of energy required to break a particular type of bond in a gaseous molecule. This value is useful in estimating the enthalpy change of a reaction by considering the energy required to break bonds in the reactants and the energy released when new bonds form in the products. While bond energies provide estimations, they do not account for all factors influencing enthalpy changes (like solvation and intermolecular forces).
Applications of Chemical Energy
The controlled release and utilization of chemical energy are crucial for numerous technological advancements:
-
Fuel Cells: Fuel cells convert the chemical energy of a fuel (like hydrogen) directly into electrical energy through electrochemical reactions, offering a cleaner alternative to combustion engines.
-
Renewable Energy: Biofuels and other renewable energy sources leverage chemical energy stored in biomass or other natural resources.
-
Materials Science: Understanding chemical bonds allows for the design and synthesis of materials with specific properties, tailored to diverse applications.
-
Medicine: Many pharmaceutical drugs function by interacting with specific molecules in the body, affecting energy-related processes.
Conclusion: The Significance of Chemical Energy
Chemical energy, the energy stored in chemical bonds, is a fundamental force driving countless processes in the universe, from the smallest biological systems to the largest industrial applications. Understanding the factors influencing energy storage in chemical bonds, the different types of bonds, and the methods of measuring energy changes are crucial for advancements in various fields. Further research and development in this area are essential for developing sustainable energy sources, designing efficient industrial processes, and fostering progress in numerous other disciplines. Harnessing and manipulating chemical energy remains a central theme in scientific and technological endeavors, promising innovation and progress in the years to come. Continued exploration of this topic will undoubtedly uncover even more of its profound implications for our world.
Latest Posts
Latest Posts
-
Does Gas Have A Definite Shape
Mar 17, 2025
-
If The Equilibrium Constant Is Negative What Does That Mean
Mar 17, 2025
-
How Does An Atom Become A Cation
Mar 17, 2025
-
Which Body Cavity Protects The Spinal Column
Mar 17, 2025
-
Sampling Distribution Of The Sample Mean Calculator
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Energy Stored In Chemical Bonds Is Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
