Enzymes Catalyze Chemical Reactions By Lowering The
Muz Play
Mar 16, 2025 · 6 min read
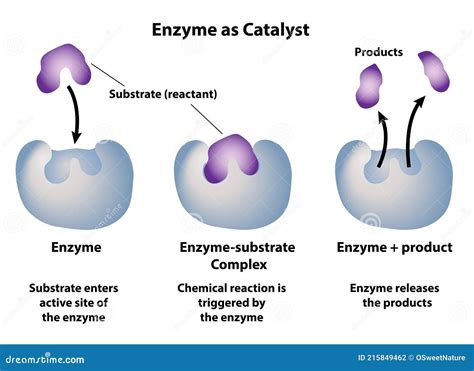
Table of Contents
Enzymes Catalyze Chemical Reactions by Lowering the Activation Energy: A Deep Dive
Enzymes are biological catalysts, crucial for virtually every metabolic process within living organisms. Their remarkable ability to accelerate chemical reactions, sometimes by factors of millions, is underpinned by their capacity to significantly lower the activation energy required for a reaction to proceed. Understanding this mechanism is fundamental to grasping the intricate workings of life itself. This article delves into the intricacies of enzyme catalysis, exploring the concept of activation energy, the diverse mechanisms employed by enzymes, and the factors that influence their activity.
Understanding Activation Energy
Before diving into the role of enzymes, it's crucial to define activation energy (Ea). Activation energy is the minimum amount of energy required for a chemical reaction to occur. Molecules need to overcome this energy barrier to reach a transition state, a high-energy intermediate state, before transforming into products. Without sufficient energy, even thermodynamically favorable reactions will proceed at an extremely slow rate, often imperceptibly so. Imagine pushing a boulder uphill; the energy required to get the boulder to the top of the hill represents the activation energy. Once it's over the crest, it rolls down the other side spontaneously.
The Activation Energy Barrier: This barrier prevents many vital biochemical reactions from occurring spontaneously at biologically relevant rates. Without enzymes, many reactions would proceed so slowly that life as we know it would be impossible.
How Enzymes Lower Activation Energy
Enzymes dramatically accelerate reaction rates by lowering the activation energy. They achieve this feat not by altering the thermodynamics of the reaction (i.e., the difference in free energy between reactants and products), but by providing an alternative reaction pathway with a lower activation energy. This is akin to creating a tunnel through the mountain, allowing the boulder to reach the other side much more easily.
Several mechanisms contribute to this reduction in activation energy:
1. Substrate Binding and Orientation:
Enzymes bind their substrates (the molecules they act upon) with high specificity at a region called the active site. This binding brings the substrates into close proximity and in the correct orientation for reaction. This precise alignment significantly increases the probability of successful collisions between reacting molecules, effectively lowering the activation energy. Imagine trying to assemble a jigsaw puzzle: arranging the pieces randomly takes much longer than having them pre-organized.
2. Strain and Distortion:
Upon binding, enzymes can induce strain or distortion in the substrate molecule. This distortion weakens bonds within the substrate, making it more susceptible to reaction. The enzyme effectively "prepares" the substrate for reaction by modifying its shape, thereby lowering the energy required for the transition state to be reached. It's like bending a branch until it's about to snap; significantly less force is then required to break it.
3. Acid-Base Catalysis:
Many enzymes utilize amino acid residues within their active sites that act as acids or bases. These residues donate or accept protons (H+), facilitating the formation or breakage of chemical bonds. The specific arrangement of these acidic and basic groups within the active site ensures precise proton transfer, thus lowering the activation energy and accelerating the reaction. This is similar to using a tool to precisely manipulate a component.
4. Covalent Catalysis:
In covalent catalysis, a temporary covalent bond forms between the enzyme and the substrate. This intermediate state can stabilize the transition state, facilitating bond breakage or formation and reducing the overall activation energy. It's analogous to using a temporary adhesive to hold two pieces together before permanently joining them.
5. Metal Ion Catalysis:
Many enzymes require metal ions (e.g., zinc, magnesium, iron) for their catalytic activity. These metal ions can participate in various ways, such as stabilizing negative charges, facilitating redox reactions (electron transfer), or directly participating in bond formation or breakage. Their presence within the active site often enhances the enzyme's ability to lower activation energy. The metal ion acts as a crucial co-factor facilitating the reaction.
Factors Affecting Enzyme Activity
The efficiency of enzyme-catalyzed reactions isn't constant; various factors can influence their activity:
1. Substrate Concentration:
At low substrate concentrations, the reaction rate increases proportionally with substrate concentration. As substrate concentration increases, the rate eventually plateaus, reaching a maximum velocity (Vmax). This occurs because all active sites on the enzyme molecules become saturated with substrate.
2. Temperature:
Enzyme activity generally increases with temperature up to a certain point (the optimum temperature). Beyond this point, high temperatures denature the enzyme, causing it to lose its three-dimensional structure and catalytic activity. The delicate balance of non-covalent interactions that maintain the enzyme's shape is disrupted by excessive heat.
3. pH:
Enzymes have an optimum pH range. Deviations from this range can alter the charge distribution within the active site, affecting substrate binding and catalytic activity. Extreme pH values can also denature the enzyme. The ionization state of crucial amino acid residues within the active site is highly pH-dependent.
4. Inhibitors:
Inhibitors are molecules that bind to enzymes and reduce their activity. Competitive inhibitors bind to the active site, competing with the substrate. Non-competitive inhibitors bind elsewhere on the enzyme, altering its conformation and reducing its catalytic efficiency. Allosteric regulators bind to regulatory sites, inducing conformational changes that affect substrate binding or catalytic activity. Inhibitors play essential roles in regulating metabolic pathways.
5. Activators:
Conversely, activators enhance enzyme activity. They can bind to the enzyme and induce a conformational change that increases its catalytic efficiency. Many coenzymes and cofactors act as activators. The interplay between inhibitors and activators fine-tunes metabolic control within cells.
Enzyme Specificity and the Active Site
The high specificity of enzyme action is a remarkable feature. This specificity arises primarily from the three-dimensional structure of the active site. The active site possesses a unique arrangement of amino acid residues and other groups that precisely complements the shape and charge distribution of the substrate. This "lock and key" model (or the more refined "induced fit" model) ensures that only the correct substrate can bind efficiently to the enzyme.
Conclusion
Enzymes are remarkable biological catalysts that accelerate chemical reactions by lowering their activation energy. Through diverse mechanisms, including substrate binding, strain and distortion, acid-base catalysis, covalent catalysis, and metal ion catalysis, they facilitate countless metabolic processes crucial for life. Understanding the factors that influence enzyme activity—substrate concentration, temperature, pH, inhibitors, and activators—is essential for comprehending the intricate regulation of biological systems. The study of enzymes continues to reveal new insights into the remarkable efficiency and specificity of biological catalysts, opening avenues for therapeutic interventions and biotechnological applications. The ability to manipulate enzyme activity holds immense promise for addressing various challenges in healthcare and industry. Further research continues to uncover new intricacies and further refine our understanding of these essential biological molecules. Their critical role in life's processes underscores the importance of continued investigation into their diverse mechanisms and regulatory influences.
Latest Posts
Latest Posts
-
Can A Buffer Be Made With A Strong Acid
Mar 17, 2025
-
Gas Laws Practice Problems With Answers
Mar 17, 2025
-
Are Strong Bases Good Leaving Groups
Mar 17, 2025
-
Which Polymer Is Composed Of Amino Acids
Mar 17, 2025
-
According To Dalton Atoms Of Different Elements Will Be
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Enzymes Catalyze Chemical Reactions By Lowering The . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
