Every Protein Has A Unique Shape And Function Because
Muz Play
Mar 15, 2025 · 6 min read
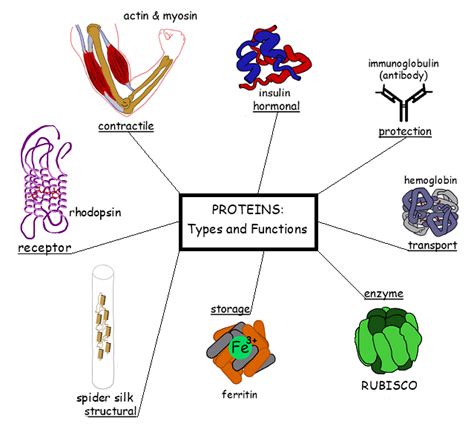
Table of Contents
Every Protein Has a Unique Shape and Function Because... The Intricate Dance of Structure and Purpose
Proteins: the workhorses of life. These complex molecules are involved in virtually every biological process, from catalyzing reactions to transporting molecules to providing structural support. But what makes each protein uniquely suited to its specific job? The answer lies in their unique three-dimensional shapes, a consequence of their intricate amino acid sequences and the interactions between them. This article delves deep into the fascinating relationship between protein structure and function, exploring the factors that determine a protein's unique conformation and how this structure dictates its biological role.
The Building Blocks: Amino Acids and the Primary Structure
The foundation of every protein's unique shape lies in its primary structure: the linear sequence of amino acids. Amino acids are the fundamental building blocks, each possessing a central carbon atom bonded to an amino group (-NH2), a carboxyl group (-COOH), a hydrogen atom, and a unique side chain (R-group). It's this R-group that gives each amino acid its distinct chemical properties – some are polar, some are nonpolar, some are charged, and some are even capable of forming disulfide bonds.
The order of these amino acids, determined by the genetic code, dictates the protein's entire architecture. A simple change in even a single amino acid can dramatically alter the protein's final shape and function. Consider sickle cell anemia, a disease caused by a single amino acid substitution in the hemoglobin protein. This seemingly minor alteration drastically changes the protein's shape, leading to impaired oxygen transport and the characteristic sickling of red blood cells.
The Importance of the Genetic Code
The genetic code, written in DNA, meticulously dictates the sequence of amino acids in a protein. This sequence is transcribed into messenger RNA (mRNA) and then translated by ribosomes into a polypeptide chain – the nascent protein. Any errors in this process, such as mutations in the DNA, can lead to alterations in the amino acid sequence and consequently, the protein's structure and function. This highlights the crucial role of DNA in ensuring the precise synthesis of functional proteins.
Shaping the Protein: Secondary, Tertiary, and Quaternary Structures
The primary amino acid sequence doesn't tell the whole story. Proteins don't exist as simple linear chains; they fold into complex three-dimensional structures, driven by various interactions between amino acid side chains. These folding patterns can be categorized into several levels of structural organization:
Secondary Structure: Local Folding Patterns
The secondary structure refers to local folding patterns stabilized by hydrogen bonds between the backbone atoms of the polypeptide chain. The most common secondary structures are:
-
α-helices: A coiled structure stabilized by hydrogen bonds between the carbonyl oxygen of one amino acid and the amide hydrogen of an amino acid four residues down the chain. This creates a rod-like structure.
-
β-sheets: Formed by hydrogen bonds between adjacent polypeptide strands, creating a pleated sheet-like structure. These strands can be parallel or antiparallel depending on the orientation of the amino acid chains.
The arrangement of α-helices and β-sheets in a protein contributes significantly to its overall shape and properties.
Tertiary Structure: The Overall 3D Conformation
The tertiary structure describes the overall three-dimensional arrangement of a polypeptide chain, encompassing all its secondary structures. This complex folding is dictated by a variety of interactions between amino acid side chains, including:
-
Hydrophobic interactions: Nonpolar side chains cluster together in the protein's interior, away from the aqueous environment.
-
Hydrogen bonds: Form between polar side chains and other polar molecules, such as water.
-
Ionic bonds (salt bridges): Electrostatic attractions between oppositely charged side chains.
-
Disulfide bonds: Covalent bonds formed between cysteine residues, creating strong cross-links within the protein.
The tertiary structure is crucial for the protein's function. It brings specific amino acid side chains into close proximity, creating the active sites of enzymes or binding sites for other molecules.
Quaternary Structure: Multiple Polypeptide Chains
Some proteins are composed of multiple polypeptide chains, each with its own tertiary structure. The arrangement of these individual subunits is called the quaternary structure. These subunits can be identical or different, and their interactions are also stabilized by the same forces that govern tertiary structure. Hemoglobin, for instance, consists of four subunits, each capable of binding oxygen. The quaternary structure allows for cooperative binding, making hemoglobin highly efficient at oxygen transport.
The Functional Consequences of Protein Shape
The unique three-dimensional shape of a protein is directly related to its function. The specific arrangement of amino acid side chains creates:
-
Active sites in enzymes: The precise three-dimensional positioning of catalytic residues in the active site ensures that the enzyme can bind its substrate and catalyze the reaction efficiently. Any alteration in the protein's shape can disrupt the active site and significantly reduce or abolish the enzyme's activity.
-
Binding sites for ligands: Proteins often bind to other molecules, such as hormones, neurotransmitters, or other proteins. The shape of the binding site determines the specificity of the interaction, ensuring that only the correct molecule binds.
-
Structural roles: Some proteins provide structural support, such as collagen in connective tissue or keratin in hair and nails. Their extended, fibrous shapes provide strength and stability.
-
Transport functions: Proteins like hemoglobin transport molecules through the body. The precise shape and binding sites allow for efficient loading and unloading of the transported molecule.
Factors Influencing Protein Folding
The process of protein folding is a complex and intricate one. Several factors influence the final three-dimensional structure:
-
Amino acid sequence: As discussed earlier, the primary structure is the primary determinant of the final folded structure.
-
Chaperone proteins: These proteins assist in the correct folding of other proteins, preventing aggregation and misfolding.
-
Environmental conditions: Factors such as temperature, pH, and ionic strength can influence protein folding. Extreme conditions can lead to protein denaturation – the unfolding and loss of function.
-
Post-translational modifications: After synthesis, proteins can undergo modifications such as glycosylation or phosphorylation, which can affect their folding and function.
Protein Misfolding and Disease
When proteins fail to fold correctly, it can lead to a variety of diseases. Misfolded proteins can aggregate, forming amyloid fibrils that are associated with neurodegenerative diseases like Alzheimer's and Parkinson's. The accumulation of these misfolded proteins disrupts cellular function and can lead to cell death. Understanding the mechanisms of protein folding and misfolding is crucial for developing therapeutic strategies to combat these diseases.
Conclusion: A Symphony of Structure and Function
The intricate relationship between protein structure and function is a remarkable testament to the elegance and efficiency of biological systems. The unique three-dimensional shape of each protein, meticulously determined by its amino acid sequence and various interactions, allows it to perform its specific biological role. Understanding this relationship is fundamental to advancing our knowledge of biology, medicine, and biotechnology. Further research into the complexities of protein folding and its impact on health and disease promises to unlock significant advancements in the treatment of various illnesses and the development of novel therapeutic strategies. The future of protein research is bright, and with each new discovery, we gain a deeper appreciation for the intricate dance of structure and purpose that underlies the very essence of life.
Latest Posts
Latest Posts
-
Do Valence Electrons Have The Most Energy
Mar 17, 2025
-
Element Vs Compound Vs Homogeneous Vs Heterogeneous
Mar 17, 2025
-
For An Exothermic Reaction The Products
Mar 17, 2025
-
Fallacies Divide Into Roughly Two Kinds
Mar 17, 2025
-
An Organism That Cannot Grow Without Oxygen Is A An
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Every Protein Has A Unique Shape And Function Because . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
