Factors Affecting E1 And E2 Reactions
Muz Play
Mar 15, 2025 · 6 min read
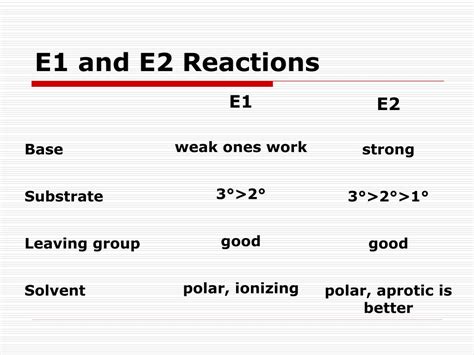
Table of Contents
Factors Affecting E1 and E2 Reactions: A Comprehensive Guide
Elimination reactions, specifically E1 and E2, are fundamental concepts in organic chemistry. Understanding the factors that influence these reactions is crucial for predicting reaction outcomes and designing synthetic strategies. This article delves deep into the intricacies of E1 and E2 reactions, exploring the various factors that govern their selectivity and efficiency. We'll examine the impact of substrate structure, leaving group, base strength and concentration, solvent, and temperature, providing a comprehensive overview for both students and seasoned chemists.
Understanding E1 and E2 Reactions: A Quick Recap
Before diving into the influencing factors, let's briefly revisit the mechanisms of E1 and E2 reactions.
E1 (Unimolecular Elimination): This two-step mechanism involves a slow, rate-determining ionization step to form a carbocation intermediate, followed by a fast elimination of a proton from a carbon adjacent to the carbocation by a base. The rate depends only on the concentration of the substrate (first-order kinetics).
E2 (Bimolecular Elimination): This concerted mechanism involves a single step where the base abstracts a proton and the leaving group departs simultaneously. The rate depends on the concentration of both the substrate and the base (second-order kinetics).
Key Factors Affecting E1 and E2 Reactions
The choice between E1 and E2 reaction pathways, and the outcome of the elimination itself (regioselectivity and stereoselectivity), are significantly influenced by several factors:
1. Substrate Structure: The Foundation of Reactivity
The structure of the alkyl halide or alcohol significantly influences the preference for E1 or E2.
a) Degree of Substitution:
-
E1: Tertiary (3°) substrates strongly favor E1 because they readily form stable carbocations. Secondary (2°) substrates can undergo both E1 and E2, while primary (1°) substrates rarely undergo E1 due to the instability of primary carbocations.
-
E2: Primary and secondary substrates are more likely to undergo E2. Tertiary substrates can undergo E2, but E1 is often the dominant pathway.
b) Steric Hindrance:
Steric bulk around the reaction center influences both mechanisms. Bulky groups near the leaving group hinder the approach of the base in E2 reactions, slowing the reaction down or changing the regioselectivity (discussed later). In E1 reactions, bulky groups can influence the stability and geometry of the carbocation intermediate.
c) Allylic and Benzylic Substrates:
Allylic and benzylic halides readily undergo elimination reactions, even under relatively mild conditions, due to the resonance stabilization of the resulting carbocation (E1) or alkene (E2).
2. Leaving Group: The Departure Agent
The leaving group's ability to depart influences the rate of both E1 and E2 reactions.
-
Good Leaving Groups: These are weak bases that readily depart as anions. Examples include halides (I⁻ > Br⁻ > Cl⁻ > F⁻), tosylate (OTs⁻), and mesylate (OMs⁻). Better leaving groups accelerate both E1 and E2 reactions.
-
Poor Leaving Groups: These are strong bases that are reluctant to depart. Hydroxide (OH⁻) and alkoxide (RO⁻) are examples of poor leaving groups. They usually require conversion to better leaving groups (e.g., through protonation) before elimination can occur.
3. Base Strength and Concentration: Driving the Reaction
The nature and concentration of the base play a crucial role in determining whether E1 or E2 will dominate.
-
E1: E1 reactions generally proceed under acidic conditions or with weak bases, and they are not significantly influenced by base concentration. The reaction proceeds via a carbocation intermediate, where the base merely acts as a proton acceptor in the second step.
-
E2: E2 reactions require a strong base. The strength of the base directly impacts the rate of the reaction. Stronger bases promote faster E2 elimination. Furthermore, the concentration of the base significantly affects the rate of E2 reactions. Higher base concentrations favor E2 over E1. Examples of strong bases commonly used in E2 reactions include potassium tert-butoxide (t-BuOK), sodium ethoxide (NaOEt), and sodium amide (NaNH₂).
4. Solvent: The Reaction Medium
The solvent can dramatically affect the rate and selectivity of elimination reactions.
-
Polar Protic Solvents: These solvents (e.g., water, alcohols) stabilize both the carbocation intermediate (in E1) and the transition state (in both E1 and E2). They are suitable for both E1 and E2 reactions, although they may favor E1 for substrates that readily form stable carbocations.
-
Polar Aprotic Solvents: These solvents (e.g., DMSO, DMF, acetone) are excellent for E2 reactions because they stabilize the negatively charged base without significantly stabilizing the leaving group. This enhances the base's nucleophilicity, leading to faster E2 rates.
5. Temperature: Kinetic Control
Temperature influences the rate of both E1 and E2 reactions. Generally, higher temperatures increase the rate of both mechanisms. However, the influence of temperature on selectivity can be more complex.
Regioselectivity and Stereoselectivity in E1 and E2 Reactions
The outcome of elimination reactions isn't just about whether E1 or E2 occurs; it also concerns the regioselectivity and stereoselectivity of the alkene product.
Regioselectivity: Choosing the Position of the Double Bond
Regioselectivity refers to the preference for formation of one alkene isomer over another.
-
E1: E1 reactions generally follow Zaitsev's rule, which states that the more substituted alkene (the one with the most alkyl groups attached to the double bond) is the major product. This is because the more substituted alkene is thermodynamically more stable.
-
E2: E2 reactions also generally follow Zaitsev's rule, but exceptions can occur with bulky bases. Bulky bases favor the less substituted alkene (Hofmann product) due to steric hindrance. The base preferentially abstracts a proton from the less hindered position, leading to the less substituted alkene.
Stereoselectivity: Controlling the Geometry of the Double Bond
Stereoselectivity refers to the preference for formation of one stereoisomer (E or Z) over the other.
-
E1: E1 reactions generally lack high stereoselectivity because the carbocation intermediate is planar, allowing the leaving group and proton to be removed from either side. A mixture of E and Z alkenes is usually observed.
-
E2: E2 reactions often exhibit high stereoselectivity. The reaction typically proceeds through an anti-periplanar transition state, where the proton and the leaving group are on opposite sides of the molecule and in the same plane. This leads to a preference for a specific alkene geometry. A syn-periplanar transition state is less common but can occur under certain conditions.
Practical Applications and Considerations
Understanding the factors influencing E1 and E2 reactions is vital in various applications, including:
-
Organic Synthesis: Choosing the right reaction conditions (substrate, base, solvent, temperature) allows chemists to selectively synthesize desired alkenes.
-
Drug Discovery: The ability to control elimination reactions is critical in the synthesis of complex molecules found in pharmaceuticals.
-
Materials Science: The design of polymers and other materials can be guided by the principles of elimination reactions.
Conclusion
The interplay of substrate structure, leaving group, base strength and concentration, solvent, and temperature significantly affects the outcome of E1 and E2 reactions. By carefully considering these factors, chemists can design experiments that favor specific elimination pathways and achieve high yields of the desired alkene products. This detailed understanding is crucial for predicting reaction outcomes and for successfully planning synthetic strategies across a wide range of organic chemistry applications. Mastering the art of controlling E1 and E2 reactions is a significant step toward proficiency in organic synthesis.
Latest Posts
Latest Posts
-
Difference Between Tlc And Column Chromatography
Mar 15, 2025
-
Energy Required To Remove An Electron From A Gaseous Atom
Mar 15, 2025
-
Que Es La Descomposicion De Acidos
Mar 15, 2025
-
Which Factor Affects Congressional Approval Ratings The Most
Mar 15, 2025
-
Fourier Transform Of A Differential Equation
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Factors Affecting E1 And E2 Reactions . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
