Half The Distance Between The Nuclei Of Two Atoms
Muz Play
Mar 17, 2025 · 7 min read
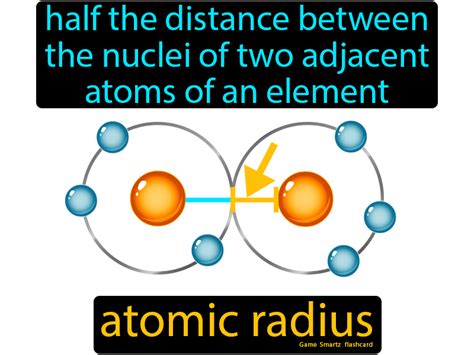
Table of Contents
Half the Distance Between the Nuclei of Two Atoms: Delving into Bond Length and its Significance
The seemingly simple concept of "half the distance between the nuclei of two atoms" actually unlocks a fundamental understanding of chemistry and material science. This distance, more formally known as half the bond length, represents a crucial parameter in describing the strength, stability, and properties of chemical bonds. This article delves deep into the significance of this seemingly small measurement, exploring its impact on various aspects of molecular structure and behavior.
Understanding Bond Length: A Foundation of Molecular Structure
The bond length, the equilibrium distance between the nuclei of two bonded atoms, is a cornerstone of molecular geometry. It's determined by a delicate balance between attractive and repulsive forces. The attractive force arises from the electrostatic interaction between the positively charged nuclei and the negatively charged electrons shared between them. Conversely, repulsive forces exist between the positively charged nuclei and between the negatively charged electrons. The bond length represents the point where these forces are in equilibrium – a point of minimum potential energy for the system.
Factors Influencing Bond Length
Several factors significantly influence the length of a chemical bond:
-
Atomic Radii: Larger atoms generally result in longer bond lengths. The size of the atoms directly affects the distance between their nuclei.
-
Bond Order: The number of electron pairs shared between two atoms (single, double, triple bonds) affects bond length. Higher bond order signifies stronger attraction, leading to shorter bond lengths. For example, a triple bond (C≡C) is shorter than a double bond (C=C), which is shorter than a single bond (C-C).
-
Hybridization: The type of hybridization of the atomic orbitals involved in bond formation impacts the bond length. Different hybrid orbitals have varying spatial distributions, influencing the effective distance between nuclei. For instance, sp hybridized orbitals lead to shorter bonds compared to sp<sup>2</sup> or sp<sup>3</sup> hybridized orbitals.
-
Electronegativity: The difference in electronegativity between the bonded atoms can affect the bond length. A greater electronegativity difference can lead to a shorter bond due to increased electron density between the nuclei. However, this effect is often secondary compared to the influence of atomic radii and bond order.
-
Resonance: In molecules exhibiting resonance, the bond length can be an average of the lengths expected for different contributing structures. The delocalization of electrons across multiple bonds results in a bond length that doesn't perfectly correspond to a single bond type.
Measuring Bond Length: Experimental Techniques
Determining the precise bond length requires sophisticated experimental techniques capable of resolving distances at the atomic level:
-
X-ray Crystallography: This widely used technique analyzes the diffraction pattern of X-rays passing through a crystalline sample. By carefully analyzing the diffraction pattern, scientists can deduce the atomic positions and calculate bond lengths.
-
Neutron Diffraction: Neutron diffraction offers advantages over X-ray diffraction for locating light atoms such as hydrogen. Neutrons interact differently with nuclei compared to X-rays, making them particularly useful for determining bond lengths involving hydrogen atoms.
-
Electron Diffraction: Similar to X-ray diffraction, this technique utilizes the diffraction of electrons to determine atomic positions and bond lengths. It's particularly suitable for studying gaseous molecules.
-
Microwave Spectroscopy: This technique analyzes the absorption and emission of microwaves by molecules. The rotational energy levels of molecules are sensitive to bond lengths, allowing for their determination from the observed microwave spectrum.
-
Spectroscopic Methods (NMR, IR): While not directly measuring bond lengths, nuclear magnetic resonance (NMR) and infrared (IR) spectroscopy provide indirect evidence about bond lengths through chemical shifts and vibrational frequencies, respectively. These spectroscopic data can be correlated with theoretical calculations to estimate bond lengths.
Significance of Bond Length in Different Contexts
The seemingly small changes in bond length have profound consequences on the physical and chemical properties of molecules and materials. Let's explore some key examples:
1. Chemical Reactivity:
Bond length directly impacts a molecule's reactivity. Shorter, stronger bonds require more energy to break, making the molecule less reactive. Conversely, longer, weaker bonds are more easily broken, increasing reactivity. This is crucial in understanding reaction mechanisms and predicting reaction rates.
2. Molecular Geometry and Shape:
Bond lengths determine the distances between atoms, ultimately defining the overall shape and geometry of a molecule. This geometry influences the molecule's polarity, intermolecular interactions, and physical properties like melting and boiling points.
3. Spectroscopic Properties:
Bond length is intimately related to the vibrational frequencies observed in infrared (IR) and Raman spectroscopy. The stronger the bond, the higher the vibrational frequency. Analyzing these frequencies provides valuable information about the types of bonds present in a molecule and their strength.
4. Material Properties:
In materials science, bond length is a critical factor determining the material's mechanical properties like hardness, strength, and elasticity. Shorter, stronger bonds contribute to higher strength and hardness. For example, the strong covalent bonds in diamond contribute to its exceptional hardness.
5. Biological Systems:
Bond lengths play a vital role in biological systems. The precise lengths of bonds in proteins and DNA dictate their three-dimensional structures, which are essential for their biological functions. Slight alterations in bond lengths can significantly impact protein folding and DNA stability.
6. Catalysis:
In catalysis, bond lengths in the active sites of catalysts are critical for their activity and selectivity. The ability of a catalyst to weaken or strengthen specific bonds in reactants is directly linked to its catalytic power. Tailoring bond lengths in catalyst design is a key area of research.
Theoretical Calculations of Bond Lengths
Computational chemistry provides powerful tools for predicting and understanding bond lengths. Quantum mechanical methods, such as Density Functional Theory (DFT) and ab initio methods, can calculate the equilibrium bond lengths with remarkable accuracy. These theoretical calculations are invaluable for studying molecules that are difficult or impossible to characterize experimentally. Comparing experimental and theoretical bond lengths helps validate the accuracy of the computational methods and our understanding of molecular interactions.
Combining Experimental and Theoretical Approaches
The most comprehensive understanding of bond lengths comes from combining both experimental and theoretical approaches. Experimental techniques provide precise measurements for specific molecules, while theoretical calculations offer insights into the factors governing bond lengths and allow predictions for molecules that are difficult to study experimentally. This integrated approach allows scientists to develop a deeper understanding of molecular structure and its relationship to chemical and physical properties.
Beyond the Basics: Advanced Concepts
The simple concept of half the distance between the nuclei of two atoms opens up a world of complexity. Exploring further, we encounter more nuanced aspects:
-
Bond Length Variations: Bond lengths are not static; they can fluctuate slightly due to vibrational motion. The average bond length is usually reported, representing the equilibrium position.
-
Anharmonic Effects: At higher temperatures, the vibrational motion becomes significant, leading to deviations from the harmonic oscillator model used in many approximations. These anharmonic effects can subtly influence the observed bond length.
-
Intramolecular Interactions: The length of one bond in a molecule can be influenced by the presence of other bonds and atoms within the same molecule. Steric effects and electronic interactions can lead to variations in bond lengths.
-
Intermolecular Interactions: Bond lengths can also be affected by interactions between molecules, particularly in condensed phases (liquids and solids). Hydrogen bonding and van der Waals forces can subtly influence the equilibrium distance between atoms.
Conclusion: The Enduring Importance of Bond Length
Half the distance between the nuclei of two atoms, a seemingly simple concept, holds a wealth of significance in chemistry, physics, and materials science. Bond length, a fundamental parameter in molecular structure, dictates reactivity, molecular geometry, spectroscopic properties, and material characteristics. The interplay between experimental techniques and theoretical calculations provides a powerful approach to understanding and predicting bond lengths, enriching our knowledge of the molecular world and its applications. Further research into the subtle nuances of bond length will undoubtedly continue to unveil new insights into the intricate relationships between molecular structure and properties.
Latest Posts
Latest Posts
-
Element Vs Compound Vs Homogeneous Vs Heterogeneous
Mar 17, 2025
-
For An Exothermic Reaction The Products
Mar 17, 2025
-
Fallacies Divide Into Roughly Two Kinds
Mar 17, 2025
-
An Organism That Cannot Grow Without Oxygen Is A An
Mar 17, 2025
-
Difference Between Chemical Reaction And Nuclear Reaction
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Half The Distance Between The Nuclei Of Two Atoms . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
