Has A Definite Volume And Shape
Muz Play
Mar 25, 2025 · 5 min read
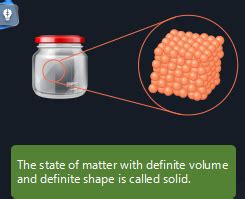
Table of Contents
Has a Definite Volume and Shape: Exploring the World of Solids
The phrase "has a definite volume and shape" is a key characteristic used to define a state of matter: the solid state. Understanding this property unlocks a deeper appreciation for the intricate world of solids, their diverse properties, and their crucial roles in our daily lives. This comprehensive article will delve into the concept of definite volume and shape, exploring the underlying reasons, exceptions, and practical implications. We will also examine how this characteristic differs from liquids and gases, and discuss various types of solids and their unique properties.
What Defines a Solid's Definite Volume and Shape?
At the heart of a solid's definite volume and shape lies the strong intermolecular forces between its constituent particles (atoms, ions, or molecules). These forces are significantly stronger than those found in liquids or gases. They hold the particles tightly together in a fixed, ordered arrangement, usually forming a crystalline lattice structure.
This rigid structure prevents the particles from moving freely. They can vibrate in place, but their overall positions remain relatively constant. This fixed arrangement dictates the solid's shape; it won't conform to the shape of its container like a liquid or gas will. Similarly, the tightly packed particles occupy a specific volume; it's nearly impossible to compress a solid significantly without applying immense pressure.
Think of it like this: Imagine a tightly packed box of Lego bricks. Each brick represents a particle. The bricks are held together, creating a fixed structure—the solid's shape. Trying to squeeze the box smaller is difficult; the bricks resist compression, representing the solid's definite volume.
The Role of Intermolecular Forces: A Deeper Dive
The strength of intermolecular forces varies widely depending on the type of solid. Covalent solids, like diamonds, are held together by strong covalent bonds, creating extremely hard and rigid structures. Ionic solids, like table salt (NaCl), are formed by the electrostatic attraction between positively and negatively charged ions, resulting in strong bonds and a definite shape. Metallic solids, such as iron or copper, consist of a "sea" of delocalized electrons surrounding positively charged metal ions, providing strong metallic bonding and a high degree of structural integrity. Finally, molecular solids, like ice, are held together by weaker intermolecular forces (e.g., hydrogen bonds, van der Waals forces), leading to varying degrees of hardness and melting points.
Exceptions to the Rule: Amorphous Solids
While most solids possess a definite volume and shape, there are exceptions. Amorphous solids, also known as non-crystalline solids, lack the ordered crystalline structure of typical solids. Their particles are arranged randomly, rather than in a regular lattice. Examples include glass, rubber, and many plastics.
Although amorphous solids maintain a definite volume, their shape can be easily altered. They lack the long-range order that provides the rigidity of crystalline solids. This is because the intermolecular forces in amorphous solids are weaker and less organized than in crystalline solids.
Understanding the Distinction: Crystalline vs. Amorphous
The distinction between crystalline and amorphous solids is fundamental. Crystalline solids exhibit long-range order, meaning their atomic arrangement repeats regularly throughout the entire solid. This leads to sharp melting points and anisotropic properties—meaning their properties vary with direction. In contrast, amorphous solids lack this long-range order, resulting in a gradual softening over a range of temperatures and isotropic properties—meaning their properties are consistent in all directions.
Comparing Solids, Liquids, and Gases
The definite volume and shape property is what truly differentiates solids from liquids and gases. Liquids have a definite volume but take the shape of their container. Their particles are close together but can move and slide past each other. Gases, on the other hand, have neither a definite volume nor shape; they expand to fill their container completely. Their particles are far apart and move freely and randomly.
A Table Summarizing the Differences
| Property | Solid | Liquid | Gas |
|---|---|---|---|
| Shape | Definite | Indefinite | Indefinite |
| Volume | Definite | Definite | Indefinite |
| Particle Arrangement | Ordered (usually) | Random, close | Random, far apart |
| Particle Movement | Vibrate in place | Slide past each other | Move freely and randomly |
| Compressibility | Low | Very low | High |
The Importance of Solids in Our World
Solids play an indispensable role in our daily lives, forming the basis of countless materials and structures. From the buildings we live in to the electronic devices we use, solids are fundamental.
Examples of Solids and Their Applications:
- Metals: Used in construction, manufacturing, and electronics due to their strength, conductivity, and malleability.
- Ceramics: Employed in cookware, insulation, and structural components due to their hardness, heat resistance, and electrical insulation properties.
- Polymers: Widely used in plastics, fabrics, and packaging due to their versatility, flexibility, and low cost.
- Semiconductors: Essential components in electronics, enabling the functioning of computers, smartphones, and other devices.
- Biomaterials: Used in medical implants and prosthetics, designed to be biocompatible and integrate with living tissue.
The Future of Materials Science: Exploring Novel Solids
Materials science continues to push the boundaries of what's possible with solids. Researchers are constantly exploring new materials with enhanced properties, focusing on:
- Nanomaterials: Solids with dimensions on the nanoscale (1-100 nanometers) exhibit unique properties, such as increased strength and reactivity.
- Metamaterials: Artificial materials engineered to have properties not found in nature, opening possibilities for advanced technologies.
- Smart materials: Solids that respond to external stimuli (temperature, pressure, light) by changing their properties. These find applications in sensors, actuators, and self-healing materials.
Conclusion: A Solid Understanding
The concept of a definite volume and shape is paramount to understanding the solid state of matter. This characteristic, rooted in the strong intermolecular forces and ordered arrangement of particles, distinguishes solids from liquids and gases. The wide variety of solid materials and their unique properties underscore their crucial role in our lives and the ongoing advancements in materials science continue to expand the possibilities of this fascinating state of matter. From the everyday objects around us to the cutting-edge technologies of the future, solids are, quite literally, the foundation upon which much of our world is built. A deeper understanding of their properties allows us to better harness their potential and innovate for a better tomorrow.
Latest Posts
Latest Posts
-
What Is The Ph Of Salt
Mar 28, 2025
-
Activity Series Of Metals And Non Metals
Mar 28, 2025
-
How Many Unpaired Electrons Does Carbon Have
Mar 28, 2025
-
What Is Damping In A Wave
Mar 28, 2025
-
How To Calculate Heat Of Dissolution Without Temperature
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Has A Definite Volume And Shape . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
