How Are Electrons Arranged Around The Nucleus
Muz Play
Mar 15, 2025 · 6 min read
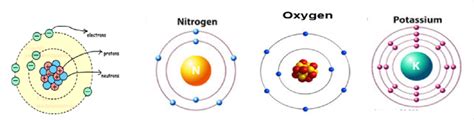
Table of Contents
How Are Electrons Arranged Around the Nucleus? Unveiling the Secrets of Atomic Structure
Understanding the arrangement of electrons around an atom's nucleus is fundamental to comprehending chemistry and the behavior of matter. This seemingly simple question opens a door to a fascinating world of quantum mechanics, orbitals, and electron configurations, shaping the properties of every element and compound in the universe. This comprehensive guide will delve deep into this topic, exploring the historical context, fundamental principles, and the implications of electron arrangement on chemical reactivity and material properties.
From Planetary Models to Quantum Mechanics: A Historical Perspective
Early models of the atom, such as the plum pudding model and the Rutherford model, provided rudimentary understandings of atomic structure. However, these models failed to accurately explain the stability of atoms and the discrete nature of atomic spectra. The limitations of classical physics became glaringly apparent.
The Bohr model, proposed in 1913, represented a significant advancement. It posited that electrons orbit the nucleus in specific energy levels or shells, each with a defined energy. Electrons could jump between these shells by absorbing or emitting photons of specific energies, explaining the discrete lines in atomic spectra. While a significant improvement, the Bohr model still had limitations, unable to account for the behavior of atoms with more than one electron.
The development of quantum mechanics revolutionized our understanding. Scientists like Schrödinger, Heisenberg, and Born developed mathematical frameworks that described the behavior of electrons not as particles orbiting the nucleus in predictable paths, but as wave-like entities existing in regions of probability called orbitals.
The Quantum Mechanical Model: Orbitals and Electron Configurations
The quantum mechanical model accurately depicts electron arrangement. Instead of distinct orbits, electrons occupy atomic orbitals, defined by four quantum numbers:
-
Principal quantum number (n): Represents the energy level or shell. It can take on positive integer values (n = 1, 2, 3...). Higher values of n indicate higher energy levels and greater distance from the nucleus.
-
Azimuthal quantum number (l): Determines the shape of the orbital and is related to the orbital angular momentum. It takes on integer values from 0 to n-1. l = 0 corresponds to an s orbital (spherical), l = 1 to a p orbital (dumbbell-shaped), l = 2 to a d orbital (more complex shapes), and l = 3 to an f orbital (even more complex shapes).
-
Magnetic quantum number (ml): Specifies the orientation of the orbital in space. It can take on integer values from -l to +l, including 0. For example, a p orbital (l = 1) has three possible orientations (ml = -1, 0, +1), often designated as px, py, and pz.
-
Spin quantum number (ms): Describes the intrinsic angular momentum of the electron, often visualized as "spin up" (ms = +1/2) or "spin down" (ms = -1/2). This is crucial for understanding the Pauli Exclusion Principle.
The Pauli Exclusion Principle and Hund's Rule
The Pauli Exclusion Principle states that no two electrons in an atom can have the same set of four quantum numbers. This means each orbital can hold a maximum of two electrons, with opposite spins.
Hund's Rule dictates that electrons will individually occupy each orbital within a subshell before doubling up in any one orbital. This minimizes electron-electron repulsion and leads to greater stability.
Filling Orbitals: Electron Configurations and the Periodic Table
The arrangement of electrons in an atom is called its electron configuration. This configuration dictates the atom's chemical properties and its position in the periodic table. The filling order generally follows the Aufbau principle, which states that electrons first fill the lowest energy levels available.
The order of filling is roughly: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p… This sequence can be remembered using mnemonic devices or by referring to a periodic table that clearly indicates the subshell filling order.
For example, the electron configuration of carbon (atomic number 6) is 1s²2s²2p². This means two electrons occupy the 1s orbital, two occupy the 2s orbital, and two occupy two of the three 2p orbitals (following Hund's Rule).
Valence Electrons and Chemical Reactivity
The valence electrons are the electrons in the outermost energy level (highest principal quantum number). These electrons are primarily responsible for an atom's chemical behavior. Atoms tend to react in ways that achieve a stable electron configuration, often by gaining, losing, or sharing valence electrons to attain a full outermost shell (usually eight electrons, the octet rule).
The Shapes of Atomic Orbitals: Visualizing Electron Density
The shapes of atomic orbitals are crucial for understanding chemical bonding.
-
s orbitals: These are spherically symmetrical, meaning the electron density is distributed uniformly in all directions around the nucleus.
-
p orbitals: These have a dumbbell shape, with two lobes of electron density on either side of the nucleus. There are three p orbitals oriented along the x, y, and z axes (px, py, pz).
-
d orbitals: These have more complex shapes, with four lobes of electron density for most of them. There are five d orbitals.
-
f orbitals: These have even more intricate shapes, with multiple lobes and regions of high electron density. There are seven f orbitals.
Hybrid Orbitals and Molecular Geometry
In many molecules, atomic orbitals combine to form hybrid orbitals. This process, called hybridization, allows for better overlap between orbitals and the formation of stronger bonds. The type of hybridization (e.g., sp, sp², sp³) influences the molecular geometry (e.g., linear, trigonal planar, tetrahedral).
Beyond the Basics: Electron Configurations and the Periodic Table's Organization
The periodic table's organization is directly related to electron configurations. Elements in the same group (vertical column) have similar valence electron configurations, leading to similar chemical properties. For instance, all alkali metals (Group 1) have one valence electron (ns¹), making them highly reactive. Transition metals have partially filled d orbitals, leading to their diverse chemical behavior and complex ion formation. The lanthanides and actinides have filling of the f orbitals, explaining their unique characteristics.
Advanced Concepts: Electron Correlation and Relativistic Effects
In larger atoms, the interactions between electrons become more complex. Electron correlation refers to the effects of electron-electron repulsion and their influence on electron distribution and energy levels. This phenomenon requires advanced computational methods to accurately predict.
Relativistic effects, significant in heavy atoms, arise from the high speeds of electrons close to the nucleus. These effects modify the electron configurations and influence chemical properties.
Conclusion: The Ever-Evolving Understanding of Atomic Structure
The arrangement of electrons around the nucleus is a cornerstone of chemistry and physics. From the early, rudimentary models to the sophisticated quantum mechanical description, our understanding has evolved dramatically. While the basic principles remain constant, the ongoing refinement of computational methods and experimental techniques continues to unveil new subtleties and complexities in atomic structure, pushing the boundaries of our understanding of the matter around us. This intricate dance of electrons dictates the properties of materials and fuels chemical reactions, shaping our world in profound ways. Continued research continues to enhance our understanding of this fundamental aspect of the universe.
Latest Posts
Latest Posts
-
How Could Sulfur Form An Ion
Mar 15, 2025
-
What Elemsnts Are Most Likey To Turn Into Anions Why
Mar 15, 2025
-
What Is The Difference Between Hunger And Appetite
Mar 15, 2025
-
Boiling Point On Graph In Celsius
Mar 15, 2025
-
List The Classification Levels From Broadest To Most Specific
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about How Are Electrons Arranged Around The Nucleus . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
