How Can You Identify A Reaction As A Redox Reaction
Muz Play
Mar 16, 2025 · 6 min read
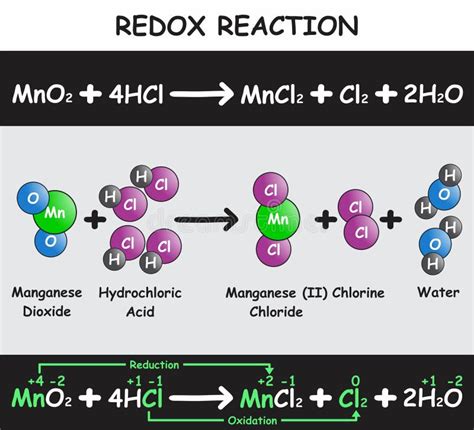
Table of Contents
How Can You Identify a Reaction as a Redox Reaction?
Redox reactions, short for reduction-oxidation reactions, are fundamental chemical processes that underpin a vast array of natural phenomena and industrial applications. From rusting iron to photosynthesis, from battery operation to combustion, redox reactions are ubiquitous. Understanding how to identify them is crucial for anyone studying chemistry, and this comprehensive guide will equip you with the knowledge and tools to confidently classify reactions.
Understanding the Fundamentals: Oxidation and Reduction
Before diving into identification methods, let's solidify our understanding of the core concepts: oxidation and reduction. These processes are always coupled; one cannot occur without the other. This is why we use the term "redox."
Oxidation: Loss of Electrons
Oxidation involves the loss of electrons by an atom, ion, or molecule. This loss results in an increase in the oxidation state (or oxidation number) of the species involved. Remember the mnemonic OIL RIG: Oxidation Is Loss, Reduction Is Gain (of electrons).
Examples:
- Fe → Fe²⁺ + 2e⁻: Iron (Fe) loses two electrons to become an iron(II) ion (Fe²⁺). Iron is oxidized.
- 2Cl⁻ → Cl₂ + 2e⁻: Two chloride ions (Cl⁻) lose two electrons to form chlorine gas (Cl₂). Chloride is oxidized.
Reduction: Gain of Electrons
Reduction involves the gain of electrons by an atom, ion, or molecule. This gain results in a decrease in the oxidation state of the species.
Examples:
- Cu²⁺ + 2e⁻ → Cu: A copper(II) ion (Cu²⁺) gains two electrons to become copper metal (Cu). Copper is reduced.
- O₂ + 4e⁻ → 2O²⁻: Oxygen gas (O₂) gains four electrons to form two oxide ions (O²⁻). Oxygen is reduced.
Key Methods for Identifying Redox Reactions
Several approaches can be employed to identify a redox reaction. Let's explore them in detail:
1. Change in Oxidation States (Oxidation Numbers)
This is the most fundamental and widely used method. By assigning oxidation numbers to each atom in the reactants and products, we can directly observe if any atom's oxidation state changes. A change in oxidation state signifies a redox reaction.
Rules for Assigning Oxidation Numbers:
- The oxidation number of an element in its free (uncombined) state is always 0. (e.g., O₂, Cl₂, Na)
- The oxidation number of a monatomic ion is equal to its charge. (e.g., Na⁺ = +1, Cl⁻ = -1)
- The oxidation number of hydrogen is usually +1, except in metal hydrides (e.g., NaH), where it is -1.
- The oxidation number of oxygen is usually -2, except in peroxides (e.g., H₂O₂), where it is -1, and in superoxides, where it has a fractional oxidation state.
- The sum of the oxidation numbers of all atoms in a neutral molecule is 0.
- The sum of the oxidation numbers of all atoms in a polyatomic ion is equal to the charge of the ion.
Example:
Consider the reaction: 2FeCl₂ + Cl₂ → 2FeCl₃
- Reactants: In FeCl₂, Fe has an oxidation state of +2, and Cl has an oxidation state of -1. In Cl₂, Cl has an oxidation state of 0.
- Products: In FeCl₃, Fe has an oxidation state of +3, and Cl has an oxidation state of -1.
Analysis: The oxidation state of Fe increases from +2 to +3 (oxidation), while the oxidation state of Cl decreases from 0 to -1 (reduction). Therefore, this is a redox reaction.
2. Identifying Oxidizing and Reducing Agents
Once you've established a redox reaction using oxidation state changes, you can identify the oxidizing and reducing agents.
- Oxidizing agent: The species that causes oxidation in another species by accepting electrons (itself being reduced).
- Reducing agent: The species that causes reduction in another species by donating electrons (itself being oxidized).
In the previous example:
- Cl₂ is the oxidizing agent because it causes Fe²⁺ to be oxidized (loses electrons) and is itself reduced.
- FeCl₂ is the reducing agent because it causes Cl₂ to be reduced (gains electrons) and is itself oxidized.
3. Presence of Electron Transfer
Though often implicit, directly observing electron transfer is a clear indicator of a redox reaction. This is particularly evident in half-reactions, which show the electron transfer explicitly.
Example:
Consider the reaction between zinc and copper(II) sulfate: Zn + CuSO₄ → ZnSO₄ + Cu
This can be broken down into half-reactions:
- Oxidation: Zn → Zn²⁺ + 2e⁻
- Reduction: Cu²⁺ + 2e⁻ → Cu
The explicit transfer of two electrons from zinc to copper(II) ions confirms this as a redox reaction.
4. Changes in the Number of Bonds to Oxygen or Hydrogen
While not as direct as oxidation state changes, changes in the number of oxygen or hydrogen atoms bonded to a central atom can often indicate a redox reaction.
- Increase in oxygen atoms: Often indicates oxidation (e.g., the combustion of methane: CH₄ + 2O₂ → CO₂ + 2H₂O)
- Decrease in oxygen atoms: Often indicates reduction.
- Increase in hydrogen atoms: Often indicates reduction (e.g., the hydrogenation of an alkene).
- Decrease in hydrogen atoms: Often indicates oxidation (e.g., the dehydrogenation of an alcohol).
Important Note: While these oxygen/hydrogen atom changes are helpful clues, they are not foolproof and should be considered alongside other methods for accurate identification.
Common Types of Redox Reactions
Several specific types of redox reactions are frequently encountered:
1. Combustion Reactions
These reactions involve the rapid reaction of a substance with oxygen, often producing heat and light. The substance being oxidized is usually a fuel (e.g., hydrocarbons), while oxygen is the oxidizing agent.
2. Corrosion Reactions
Corrosion, such as rusting, is a redox process where a metal reacts with its environment (usually oxygen and water) to form metal oxides or hydroxides. The metal is oxidized, and oxygen is reduced.
3. Single Displacement Reactions
These reactions involve a more reactive element displacing a less reactive element from a compound. The more reactive element is oxidized, and the less reactive element is reduced. For example, the reaction between zinc and hydrochloric acid: Zn + 2HCl → ZnCl₂ + H₂
4. Disproportionation Reactions
In this type of redox reaction, a single species undergoes both oxidation and reduction simultaneously. For example, the decomposition of hydrogen peroxide: 2H₂O₂ → 2H₂O + O₂
5. Redox Titrations
These are quantitative analytical techniques used to determine the concentration of an unknown substance using a redox reaction. A known volume of a solution of known concentration (the titrant) is added to a solution of the unknown concentration until the reaction is complete, typically signaled by a color change.
Avoiding Common Mistakes in Redox Reaction Identification
Even with the methods described above, there are potential pitfalls to avoid:
- Incomplete Oxidation State Assignments: Carefully follow the rules for assigning oxidation numbers. Overlooking certain elements or applying rules incorrectly will lead to inaccurate conclusions.
- Ignoring Spectator Ions: Spectator ions (ions that don't participate in the redox reaction) should be ignored when analyzing oxidation state changes.
- Confusing Oxidation and Reduction: Remember OIL RIG – Oxidation Is Loss, Reduction Is Gain. Always clearly identify which species is losing electrons and which is gaining.
- Relying Solely on Oxygen/Hydrogen Changes: As mentioned, while helpful, changes in oxygen or hydrogen bonding alone are not sufficient to definitively confirm a redox reaction.
Conclusion: Mastering Redox Reaction Identification
Identifying redox reactions is a fundamental skill in chemistry. By mastering the techniques described – focusing primarily on changes in oxidation states, but also utilizing the other methods and avoiding common errors – you can confidently classify reactions and deepen your understanding of this crucial chemical process. Remember to always systematically analyze the reactants and products, apply the rules of oxidation number assignment meticulously, and consider the overall context of the reaction. With practice, identifying redox reactions will become second nature.
Latest Posts
Latest Posts
-
Can A Buffer Be Made With A Strong Acid
Mar 17, 2025
-
Gas Laws Practice Problems With Answers
Mar 17, 2025
-
Are Strong Bases Good Leaving Groups
Mar 17, 2025
-
Which Polymer Is Composed Of Amino Acids
Mar 17, 2025
-
According To Dalton Atoms Of Different Elements Will Be
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about How Can You Identify A Reaction As A Redox Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
