How Does A Catalyst Influence A Chemical Reaction
Muz Play
Mar 21, 2025 · 6 min read
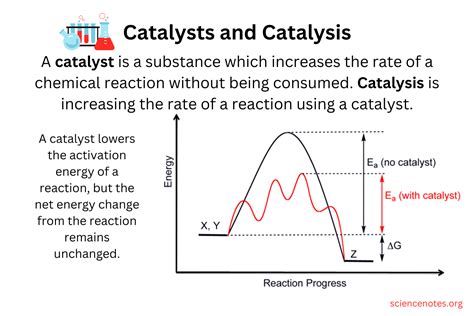
Table of Contents
How Does a Catalyst Influence a Chemical Reaction?
A catalyst is a substance that speeds up the rate of a chemical reaction without being consumed in the process. Understanding how catalysts achieve this is fundamental to chemistry and has vast implications across numerous fields, from industrial manufacturing to biological processes. This article delves deep into the mechanics of catalysis, exploring its different types, mechanisms, and crucial role in shaping our world.
The Essence of Catalysis: Lowering Activation Energy
The core function of a catalyst is to reduce the activation energy of a chemical reaction. Activation energy is the minimum amount of energy required for reactants to transform into products. Think of it as the energy barrier that needs to be overcome for a reaction to proceed. A catalyst achieves this reduction by providing an alternative reaction pathway with a lower activation energy. This doesn't alter the overall enthalpy change (ΔH) of the reaction—the difference in energy between reactants and products remains the same—but it significantly accelerates the rate at which the reaction occurs.
Visualizing the Energy Landscape
Imagine a ball rolling down a hill. The hill represents the energy profile of a reaction. Without a catalyst, the ball has to roll over a high peak (high activation energy) to reach the bottom (products). A catalyst essentially creates a shorter, less steep path, allowing the ball to reach the bottom much faster, even though the overall drop in height (ΔH) remains unchanged.
Mechanisms of Catalysis: A Closer Look
The exact mechanism by which a catalyst lowers activation energy varies depending on the specific catalyst and reaction. However, some common mechanisms include:
1. Adsorption and Surface Reactions: Heterogeneous Catalysis
Heterogeneous catalysis involves catalysts in a different phase from the reactants. A common example is the use of solid catalysts in gas-phase reactions. In this case, the reactants are adsorbed onto the catalyst's surface, forming a temporary bond. This adsorption weakens the bonds within the reactant molecules, making them more susceptible to reaction. The reaction then occurs on the surface, and the products subsequently desorb, freeing the catalyst surface for further reactions.
Key aspects of heterogeneous catalysis:
- Surface area: A larger surface area of the catalyst allows for more reactant adsorption, increasing the reaction rate. This is why catalysts are often finely divided or porous.
- Active sites: Specific locations on the catalyst surface with unique electronic properties are responsible for the catalytic activity. These are known as active sites.
- Selectivity: Catalysts can often be designed to favor the formation of specific products, enhancing the selectivity of the reaction.
2. Formation of Intermediates: Homogeneous Catalysis
Homogeneous catalysis involves catalysts in the same phase as the reactants. These catalysts typically interact directly with the reactants to form intermediates, which then react further to produce the desired products. The catalyst is regenerated in the process, allowing it to participate in multiple reaction cycles.
Examples of homogeneous catalysts:
- Transition metal complexes: Many transition metal complexes act as homogeneous catalysts, facilitating reactions like oxidation, reduction, and carbonylation.
- Enzymes: Enzymes are biological catalysts that function through the formation of enzyme-substrate complexes. These complexes stabilize the transition state, lowering the activation energy.
3. Acid-Base Catalysis
Acid-base catalysis involves the use of acids or bases to either donate or accept protons (H+), influencing the reaction pathway. The catalyst facilitates the reaction by either protonating or deprotonating a reactant, making it more reactive. This mechanism is particularly prevalent in organic reactions.
Types of Catalysts: A Diverse Landscape
Catalysts come in various forms, each tailored to specific applications:
1. Metal Catalysts: Powerhouses of Industry
Metal catalysts, particularly transition metals like platinum, palladium, nickel, and rhodium, are widely used in industrial processes. Their ability to form various oxidation states and coordinate with reactants makes them versatile in a range of reactions, including hydrogenation, oxidation, and polymerization.
2. Enzyme Catalysts: Nature's Masterpieces
Enzymes are biological catalysts that exhibit remarkable specificity and efficiency. Their intricate three-dimensional structures create active sites that precisely bind to specific substrates, facilitating highly selective reactions with astounding speed under mild conditions.
3. Zeolites: Porous Wonders
Zeolites are microporous aluminosilicate minerals with a highly ordered crystalline structure. Their extensive network of pores and channels creates a large surface area, making them effective catalysts for various reactions, especially in petroleum refining and petrochemical industries.
Factors Affecting Catalytic Activity
Several factors influence the efficiency and selectivity of a catalyst:
1. Temperature: A Delicate Balance
Increasing the temperature generally increases the reaction rate, but excessively high temperatures can deactivate some catalysts. Finding the optimal temperature is crucial for maximizing catalytic efficiency.
2. Pressure: Influencing Reactant Concentration
Pressure affects the concentration of gaseous reactants adsorbed on the catalyst surface in heterogeneous catalysis. Higher pressure usually leads to higher adsorption and reaction rates, but excessive pressure can also affect catalyst stability.
3. Catalyst Concentration: More Isn't Always Better
While increasing catalyst concentration usually speeds up the reaction, a point of diminishing returns can be reached. Beyond a certain concentration, further increases may not significantly affect the reaction rate.
4. Presence of Inhibitors and Poisons: Catalyst Deactivation
Inhibitors and poisons are substances that interfere with the catalytic activity. They can bind to active sites, blocking reactant access and reducing the catalyst's effectiveness. Understanding and mitigating the effects of inhibitors and poisons are critical in maintaining catalyst performance.
Applications of Catalysis: Shaping Our World
Catalysis plays a vital role in countless applications across diverse fields:
1. Industrial Chemistry: Driving Production
Catalysis underpins many large-scale industrial processes, such as the Haber-Bosch process for ammonia synthesis, the production of sulfuric acid, and the cracking of petroleum to produce gasoline. These processes rely heavily on catalysts to achieve high yields and efficiency.
2. Automotive Industry: Controlling Emissions
Catalytic converters in automobiles utilize metal catalysts to convert harmful exhaust gases like carbon monoxide and nitrogen oxides into less harmful substances like carbon dioxide and nitrogen. This technology plays a crucial role in reducing air pollution.
3. Pharmaceuticals: Synthesizing Drugs
Catalysis is essential in the synthesis of many pharmaceutical drugs. Specific catalysts facilitate the creation of complex molecules with high selectivity, ensuring the production of pure and effective medicines.
4. Biology: Enabling Life
Enzymes are biological catalysts that drive virtually all biochemical reactions within living organisms. They control metabolic processes, DNA replication, and countless other essential functions, making them indispensable for life itself.
Conclusion: The Unseen Force Driving Chemical Reactions
Catalysts are the unsung heroes of chemistry, quietly influencing the speed and direction of countless chemical reactions. Understanding their mechanisms, types, and applications is paramount in many scientific and technological endeavors. The ongoing development of new and improved catalysts promises to continue shaping our world, driving innovations in various fields and addressing pressing global challenges. From optimizing industrial processes to developing life-saving drugs and combating pollution, the impact of catalysts is undeniable and far-reaching. The exploration of catalysis continues to be a vibrant area of research, unveiling new possibilities and expanding our understanding of this fundamental phenomenon.
Latest Posts
Latest Posts
-
Five Number Summary And Box Plot
Mar 21, 2025
-
Interpersonal Communication A Mindful Approach To Relationships
Mar 21, 2025
-
How Do Nonsteroid Hormones Differ From Steroid Hormones
Mar 21, 2025
-
Temperature Is Proportional To The Kinetic Energy
Mar 21, 2025
-
What Is A Property Of A Solid
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about How Does A Catalyst Influence A Chemical Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
