How Does A Spectrophotometer Measure Absorbance
Muz Play
Mar 28, 2025 · 7 min read
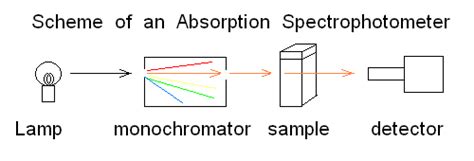
Table of Contents
How Does a Spectrophotometer Measure Absorbance? A Deep Dive
Spectrophotometry is a fundamental technique in various scientific fields, from chemistry and biochemistry to environmental science and materials science. At its core, it's a method for measuring the amount of light absorbed by a sample at specific wavelengths. This measurement, known as absorbance, provides crucial information about the sample's concentration, purity, and other properties. But how exactly does a spectrophotometer achieve this precise measurement? This article will delve into the intricate workings of a spectrophotometer, explaining the principles behind absorbance measurement and the different types of spectrophotometers available.
Understanding the Fundamentals: Beer-Lambert Law
The cornerstone of spectrophotometry is the Beer-Lambert Law, also known as the Beer-Lambert-Bouguer Law. This law mathematically relates the absorbance of a solution to its concentration and the path length of the light beam through the solution. The equation is:
A = εbc
Where:
- A represents absorbance (unitless)
- ε represents the molar absorptivity (L mol⁻¹ cm⁻¹) – a constant specific to the substance and the wavelength of light used. It represents how strongly the substance absorbs light at a particular wavelength.
- b represents the path length (cm) – the distance the light travels through the sample. This is typically the width of the cuvette holding the sample.
- c represents the concentration (mol L⁻¹) of the substance in the solution.
This equation highlights the direct proportionality between absorbance and both concentration and path length. Double the concentration, and you double the absorbance (at a constant path length and wavelength). Double the path length, and you double the absorbance (at a constant concentration and wavelength). This relationship allows us to use a spectrophotometer to determine the concentration of an unknown sample by comparing its absorbance to that of a known standard.
The Components of a Spectrophotometer: A Detailed Look
A spectrophotometer, regardless of its type, consists of several key components working in concert to measure absorbance:
1. Light Source: Illuminating the Sample
The light source provides the electromagnetic radiation (light) that will interact with the sample. Common light sources include:
- Tungsten Filament Lamps: Produce a continuous spectrum of visible light, suitable for measuring absorbance in the visible region (approximately 380-750 nm).
- Deuterium Lamps: Emit a continuous spectrum of ultraviolet (UV) light, crucial for measuring absorbance in the UV region (typically 190-400 nm).
- Xenon Lamps: Provide a broad spectrum covering both UV and visible regions, offering versatility in applications.
- LEDs: Increasingly used, LEDs offer advantages such as long lifespan, low power consumption, and monochromatic output (emitting light at a specific wavelength).
The choice of light source depends on the wavelength range of interest for the specific application.
2. Monochromator: Selecting the Wavelength
The monochromator isolates specific wavelengths of light from the continuous spectrum produced by the light source. This is crucial because the absorbance of a substance varies with wavelength, exhibiting characteristic peaks and troughs in its absorbance spectrum. Common monochromator types include:
- Prisms: Separate light based on its refractive index, bending different wavelengths at different angles.
- Diffraction Gratings: Consist of closely spaced parallel lines that diffract light, separating it into its component wavelengths.
The monochromator allows the user to select the desired wavelength for measurement.
3. Sample Holder (Cuvette): Containing the Sample
The sample holder, typically a cuvette, is a small transparent container that holds the sample to be analyzed. Cuvettes are usually made of quartz or glass, depending on the wavelength range. Quartz is essential for UV measurements as glass absorbs UV light. The path length of the cuvette (typically 1 cm) is a crucial parameter in the Beer-Lambert Law. The cuvette is carefully placed in the sample holder, ensuring correct alignment with the light beam.
4. Detector: Measuring the Transmitted Light
After passing through the sample, the remaining light (transmitted light) is detected by a photodetector. This device measures the intensity of the light that passed through the sample. Common detectors include:
- Photomultiplier Tubes (PMTs): Highly sensitive detectors that amplify the light signal, making them ideal for low-intensity light measurements.
- Photodiodes: Solid-state detectors that are less sensitive than PMTs but more robust and less expensive.
The detector converts the light intensity into an electrical signal, which is then processed by the spectrophotometer's electronics.
5. Readout Device: Displaying the Results
The readout device displays the measured absorbance, usually as a numerical value on a screen. Modern spectrophotometers often offer additional features, such as data storage, spectral scanning capabilities, and software for data analysis.
The Measurement Process: Step-by-Step
The measurement process typically involves the following steps:
-
Wavelength Selection: The desired wavelength is selected using the monochromator.
-
Blank Measurement: A blank solution (a solution containing everything except the analyte) is measured to establish a baseline. This corrects for any absorbance by the solvent or cuvette.
-
Sample Measurement: The sample solution is measured at the selected wavelength.
-
Absorbance Calculation: The spectrophotometer calculates the absorbance using the measured intensities of the blank and the sample. The absorbance is calculated as:
A = log₁₀(I₀/I)
where:
- I₀ is the intensity of light passing through the blank (reference).
- I is the intensity of light passing through the sample.
-
Data Analysis: The obtained absorbance is then used to determine the concentration of the analyte using the Beer-Lambert Law, often by creating a calibration curve using solutions of known concentrations.
Types of Spectrophotometers: Exploring the Variations
Spectrophotometers come in various forms, each optimized for specific applications:
1. Single-Beam Spectrophotometers: Simplicity and Affordability
Single-beam spectrophotometers measure the absorbance of a sample by measuring the intensity of light passing through the sample and then comparing it to a blank measurement. They are relatively simple and affordable but require measuring the blank and sample separately.
2. Double-Beam Spectrophotometers: Enhanced Accuracy and Speed
Double-beam spectrophotometers split the light beam into two paths: one path passes through the sample, and the other path passes through a blank. The instrument simultaneously measures the intensity of light through both paths, providing continuous monitoring and increased accuracy. They are faster and more precise than single-beam spectrophotometers, but generally more expensive.
3. UV-Vis Spectrophotometers: Covering the UV and Visible Regions
UV-Vis spectrophotometers are the most common type, covering both the ultraviolet and visible regions of the electromagnetic spectrum (approximately 190-800 nm). They are versatile and used in various applications, including quantitative analysis, qualitative analysis (identifying substances based on their absorption spectra), and kinetic studies (monitoring reaction rates).
4. Atomic Absorption Spectrophotometers (AAS): Analyzing Elemental Composition
AAS is a specialized technique used for determining the concentration of specific elements in a sample. It utilizes a flame or graphite furnace to atomize the sample, and a light source emitting the characteristic wavelength of the element being analyzed. The absorbance of the atomic vapor is then measured to determine the element's concentration.
Applications of Spectrophotometry: A Wide Range of Uses
The applications of spectrophotometry are incredibly diverse and span various scientific disciplines:
- Clinical Chemistry: Measuring glucose, cholesterol, and other analytes in blood samples.
- Biochemistry: Determining the concentration of proteins, DNA, and RNA.
- Environmental Science: Monitoring pollutants in water and air.
- Food Science: Analyzing the color and composition of food products.
- Pharmaceutical Industry: Quality control of drugs and medications.
- Materials Science: Characterizing the properties of materials.
Conclusion: A Powerful Tool for Scientific Analysis
Spectrophotometry is a powerful and versatile analytical technique that allows for precise measurement of light absorbance, providing valuable insights into the properties of various samples. Understanding the underlying principles of the Beer-Lambert Law and the components of a spectrophotometer are crucial for successful applications of this technique. The diverse range of spectrophotometer types and their wide-ranging applications solidify spectrophotometry's position as a cornerstone of modern scientific research and analysis. Its continued development and refinement will undoubtedly lead to further advancements and applications across numerous fields.
Latest Posts
Latest Posts
-
Polynomial Long Division Worksheet With Answers Pdf
Mar 31, 2025
-
What Is The Difference Between A Law And A Theory
Mar 31, 2025
-
Is Cn A Good Leaving Group
Mar 31, 2025
-
Equations For Motion With Constant Acceleration
Mar 31, 2025
-
Double Replacement Reaction Examples In Real Life
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about How Does A Spectrophotometer Measure Absorbance . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
