How Is Energy Involved In Chemical And Physical Changes
Muz Play
Mar 31, 2025 · 6 min read
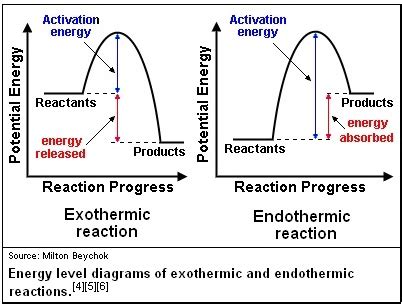
Table of Contents
How is Energy Involved in Chemical and Physical Changes?
Energy plays a pivotal role in both chemical and physical changes, acting as the driving force behind transformations in matter. Understanding this fundamental connection is key to comprehending the world around us, from the rusting of iron to the boiling of water. This article delves deep into the intricate relationship between energy and matter transformations, exploring the nuances of exothermic and endothermic processes, activation energy, and the various forms of energy involved.
The Fundamentals: Energy and Its Forms
Before diving into the specifics of chemical and physical changes, let's establish a foundational understanding of energy. Energy, in its simplest form, is the capacity to do work or cause change. It exists in various forms, including:
-
Kinetic Energy: The energy of motion. Anything that's moving possesses kinetic energy – from a speeding car to the vibrating atoms within a substance. The faster the motion, the greater the kinetic energy.
-
Potential Energy: Stored energy due to an object's position or configuration. A stretched rubber band, a book held above the ground, and chemical bonds all possess potential energy. This stored energy can be released and converted into other forms of energy.
-
Thermal Energy (Heat): The total kinetic energy of the particles within a substance. Temperature is a measure of the average kinetic energy of these particles.
-
Chemical Energy: Potential energy stored within the bonds of molecules. The breaking and forming of chemical bonds involve the release or absorption of chemical energy.
-
Electrical Energy: Energy associated with the flow of electric charge.
-
Radiant Energy (Light): Energy that travels in the form of electromagnetic waves.
Energy in Physical Changes
Physical changes alter the form or appearance of matter without changing its chemical composition. These changes often involve energy transfer, but they don't create new substances. Examples include:
Phase Changes:
Phase changes, such as melting, freezing, boiling, and condensation, are prime examples of energy's role in physical changes.
-
Melting (Solid to Liquid): Requires energy input (endothermic) to overcome the intermolecular forces holding the solid together. This energy increases the kinetic energy of the particles, allowing them to move more freely and transition to the liquid phase.
-
Freezing (Liquid to Solid): Releases energy (exothermic) as the particles lose kinetic energy and the intermolecular forces pull them closer together, forming a solid structure.
-
Boiling (Liquid to Gas): Requires significant energy input (endothermic) to overcome the strong intermolecular forces in the liquid and allow particles to escape into the gaseous phase.
-
Condensation (Gas to Liquid): Releases energy (exothermic) as gas particles lose kinetic energy and come closer together, forming liquid droplets.
Dissolving:
Dissolving a substance in a solvent, like sugar in water, is another physical change. The process can be either endothermic or exothermic, depending on the specific solute and solvent. Energy is involved in breaking the intermolecular forces within the solute and between the solvent molecules, and in forming new interactions between the solute and solvent particles.
Changes in Shape and Size:
Crushing a can, bending a wire, or cutting paper are all physical changes that involve energy transfer. Energy is required to overcome the forces holding the material in its original shape. While the chemical composition remains unchanged, the arrangement of particles is altered.
Energy in Chemical Changes
Chemical changes, also known as chemical reactions, involve the rearrangement of atoms and the formation of new substances with different properties. These changes are always accompanied by energy changes.
Exothermic Reactions:
Exothermic reactions release energy into their surroundings, often in the form of heat or light. The products of the reaction have less potential energy than the reactants. Examples include:
-
Combustion: Burning fuels, such as wood or gasoline, releases a significant amount of heat and light energy.
-
Neutralization Reactions: The reaction between an acid and a base releases heat.
-
Respiration: The process by which living organisms convert food into energy is an exothermic reaction.
Endothermic Reactions:
Endothermic reactions absorb energy from their surroundings. The products of the reaction have more potential energy than the reactants. Examples include:
-
Photosynthesis: Plants absorb light energy to convert carbon dioxide and water into glucose and oxygen.
-
Electrolysis: The decomposition of water into hydrogen and oxygen using electrical energy.
-
Melting Ice: As discussed earlier, melting ice is an endothermic process requiring energy input to break the bonds holding the water molecules in a solid structure. While this is a physical change on a macroscopic level, at a microscopic level, the hydrogen bonds are being broken and reformed, making it a chemical change.
Activation Energy: The Energy Barrier
Regardless of whether a reaction is exothermic or endothermic, it requires a certain amount of energy to initiate it. This is known as activation energy. Activation energy is the minimum energy required to break the existing bonds in the reactants and initiate the reaction. Once the activation energy is reached, the reaction can proceed, either releasing or absorbing energy.
Think of it like pushing a boulder uphill. You need to exert energy (activation energy) to get the boulder moving. Once it starts rolling downhill, it releases energy. Similarly, in an exothermic reaction, the energy released is greater than the activation energy. In an endothermic reaction, the energy absorbed is greater than the activation energy.
Measuring Energy Changes: Enthalpy and Calorimetry
The energy change in a chemical or physical process is often quantified using enthalpy (ΔH). Enthalpy is a measure of the total heat content of a system at constant pressure. A negative ΔH indicates an exothermic reaction (heat released), while a positive ΔH indicates an endothermic reaction (heat absorbed).
Calorimetry is a technique used to experimentally determine the enthalpy change of a reaction. A calorimeter is a device that measures the heat transferred during a reaction.
Energy and the Laws of Thermodynamics
The relationship between energy and matter transformations is governed by the laws of thermodynamics. These laws dictate the direction and extent of energy changes in physical and chemical processes:
-
The First Law of Thermodynamics (Law of Conservation of Energy): Energy cannot be created or destroyed, only transformed from one form to another. The total energy of a system and its surroundings remains constant.
-
The Second Law of Thermodynamics: The total entropy (disorder) of a system and its surroundings always increases in a spontaneous process. This law explains why some processes occur spontaneously while others do not.
Applications and Real-World Examples
The principles of energy transfer in physical and chemical changes are fundamental to numerous applications and processes:
-
Power Generation: Burning fossil fuels in power plants converts chemical energy into thermal energy, which is then used to generate electricity.
-
Food Preservation: Freezing food slows down chemical reactions that cause spoilage, preserving its quality and preventing bacterial growth.
-
Industrial Processes: Many industrial processes, such as the production of metals and chemicals, rely on controlled chemical reactions that involve significant energy changes.
-
Medicine: Many medical treatments, such as chemotherapy and radiation therapy, utilize energy to target and destroy cancer cells.
Conclusion
The interplay between energy and matter is a cornerstone of chemistry and physics. Understanding the different forms of energy, the energy changes involved in chemical and physical processes, and the principles of thermodynamics is crucial for comprehending a vast range of phenomena in the natural world and technological applications. From the simplest phase change to the most complex chemical reactions, energy is the driving force that shapes our universe. By understanding this fundamental connection, we can better appreciate the intricate workings of the world around us and harness the power of energy for beneficial purposes.
Latest Posts
Latest Posts
-
The Properties Of Life Mastering Biology
Apr 01, 2025
-
Effective Nuclear Charge Periodic Table Trend
Apr 01, 2025
-
Examples Of Instantaneous Rate Of Change
Apr 01, 2025
-
How Is The Use Of Symbols Related To Culture
Apr 01, 2025
-
As You Move Across The Periodic Table
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about How Is Energy Involved In Chemical And Physical Changes . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
