How Many Bonds Can Each Element Have Lewis Structure
Muz Play
Mar 15, 2025 · 6 min read
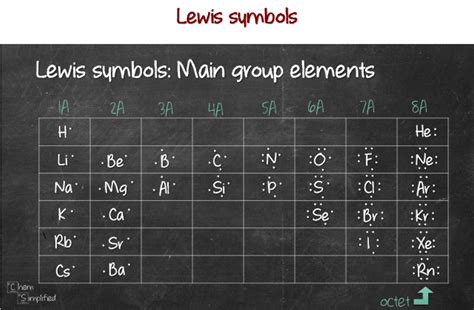
Table of Contents
How Many Bonds Can Each Element Have? A Deep Dive into Lewis Structures and Bonding Capacity
Understanding how many bonds an element can form is crucial for predicting molecular geometry, reactivity, and various other chemical properties. This ability is directly linked to an element's valence electrons and its position within the periodic table. This comprehensive guide delves into the intricacies of Lewis structures and explains the bonding capacity of various elements, focusing on the interplay between valence electrons, octet rule, and exceptions.
Understanding Lewis Structures and Valence Electrons
Before we explore the bonding capacity of individual elements, let's establish a solid foundation in Lewis structures and valence electrons. A Lewis structure, also known as an electron dot diagram, is a visual representation of the valence electrons in an atom or molecule. Valence electrons are the electrons residing in the outermost shell of an atom and are responsible for chemical bonding. These electrons are depicted as dots surrounding the element's symbol.
The number of valence electrons an atom possesses directly determines its bonding capacity. Elements strive to achieve a stable electron configuration, typically resembling a noble gas (Group 18). This is often achieved by gaining, losing, or sharing electrons to obtain a full outermost electron shell. This principle is often referred to as the octet rule, which states that atoms tend to gain, lose, or share electrons to achieve eight electrons in their valence shell. However, it's crucial to remember that this is a guideline, not an absolute law, and exceptions exist.
Predicting Valence Electrons
The number of valence electrons can be easily predicted from an element's group number in the periodic table (excluding transition metals):
- Group 1 (Alkali Metals): 1 valence electron
- Group 2 (Alkaline Earth Metals): 2 valence electrons
- Group 13 (Boron Group): 3 valence electrons
- Group 14 (Carbon Group): 4 valence electrons
- Group 15 (Pnictogens): 5 valence electrons
- Group 16 (Chalcogens): 6 valence electrons
- Group 17 (Halogens): 7 valence electrons
- Group 18 (Noble Gases): 8 valence electrons (except helium, which has 2)
Bonding Capacity and the Octet Rule: A Detailed Look at Different Elements
Now, let's explore the bonding capacity of different elements, keeping in mind the octet rule and its exceptions.
Group 14: Carbon Family (C, Si, Ge, Sn, Pb)
Elements in Group 14 have four valence electrons. To achieve a stable octet, they typically form four covalent bonds. Carbon (C) is a prime example; it forms four single bonds (e.g., methane, CH₄), two double bonds (e.g., carbon dioxide, CO₂), or one triple bond and one single bond (e.g., hydrogen cyanide, HCN). Silicon (Si), Germanium (Ge), Tin (Sn), and Lead (Pb) also exhibit a tendency towards forming four bonds, although the stability and prevalence of higher coordination numbers increase as you move down the group.
Group 15: Pnictogens (N, P, As, Sb, Bi)
Group 15 elements possess five valence electrons. They typically form three covalent bonds to achieve an octet, though they can participate in other bonding scenarios. Nitrogen (N), for example, forms three single bonds in ammonia (NH₃) or one triple bond in nitrogen gas (N₂). Phosphorus (P), Arsenic (As), Antimony (Sb), and Bismuth (Bi) also show a preference for forming three bonds but can expand their octet under certain circumstances to accommodate more bonds.
Group 16: Chalcogens (O, S, Se, Te, Po)
Group 16 elements have six valence electrons. They commonly form two covalent bonds to complete their octet. Oxygen (O), for instance, forms two single bonds in water (H₂O) or a double bond in carbon dioxide (CO₂). Sulfur (S), Selenium (Se), Tellurium (Te), and Polonium (Po) also generally form two bonds but can exhibit higher coordination numbers due to their ability to expand their octet (d-orbital participation).
Group 17: Halogens (F, Cl, Br, I, At)
Halogens have seven valence electrons and usually form one single covalent bond to achieve a stable octet. Fluorine (F), Chlorine (Cl), Bromine (Br), Iodine (I), and Astatine (At) all commonly participate in this type of bonding.
Group 1: Alkali Metals (Li, Na, K, Rb, Cs, Fr)
Alkali metals possess one valence electron. They tend to lose this electron to form a +1 cation, achieving a stable electron configuration. They rarely form covalent bonds.
Group 2: Alkaline Earth Metals (Be, Mg, Ca, Sr, Ba, Ra)
Alkaline earth metals have two valence electrons and usually lose both electrons to form a +2 cation, also achieving a noble gas configuration. Covalent bonding is less prevalent compared to ionic bonding.
Exceptions to the Octet Rule
While the octet rule serves as a helpful guideline, several exceptions exist:
- Electron-deficient compounds: Some compounds, like boron trifluoride (BF₃), have fewer than eight electrons around the central atom (boron in this case). Boron only has six valence electrons in BF₃.
- Odd-electron compounds: Molecules with an odd number of valence electrons, like nitrogen dioxide (NO₂), cannot satisfy the octet rule for all atoms.
- Hypervalent compounds: Some elements, particularly those in the third period and beyond, can expand their octet and accommodate more than eight electrons in their valence shell. This is common for elements like phosphorus (P) and sulfur (S) and is attributed to the availability of empty d-orbitals for bonding. Examples include phosphorus pentachloride (PCl₅) and sulfur hexafluoride (SF₆).
- Hydrogen: Hydrogen only needs two electrons to achieve a stable configuration (duet rule) resembling helium.
Determining Bond Order and Resonance Structures
The Lewis structure provides a basis for determining the bond order, which represents the number of chemical bonds between a pair of atoms. A single bond has a bond order of 1, a double bond has a bond order of 2, and a triple bond has a bond order of 3. In molecules exhibiting resonance, the bond order is an average of the bond orders in the contributing resonance structures. Resonance occurs when a molecule can be represented by two or more Lewis structures that differ only in the placement of electrons.
Advanced Concepts and Applications
The principles discussed above are fundamental to understanding chemical bonding. However, several advanced concepts build upon this foundation:
- Molecular Orbital Theory: This theory provides a more sophisticated understanding of bonding, describing molecular orbitals formed by the combination of atomic orbitals.
- Formal Charge: This concept helps to determine the most likely Lewis structure among several possibilities by assigning formal charges to atoms in a molecule.
- VSEPR Theory (Valence Shell Electron Pair Repulsion Theory): This theory predicts the three-dimensional geometry of molecules based on the arrangement of electron pairs around the central atom.
- Hybridization: This concept explains the mixing of atomic orbitals to form hybrid orbitals with different shapes and energies. These hybrid orbitals are then used to form sigma and pi bonds.
Conclusion
Predicting the number of bonds an element can form is a cornerstone of chemistry. While the octet rule provides a valuable framework, exceptions exist, highlighting the complexities of chemical bonding. A comprehensive understanding of Lewis structures, valence electrons, and the various exceptions allows for accurate prediction of molecular properties and reactivity. This knowledge is essential for further exploration of advanced concepts in chemistry, such as molecular orbital theory, VSEPR theory, and hybridization. By understanding the interplay between valence electrons and the tendency towards a stable electronic configuration, we can unlock a deeper comprehension of the fundamental forces shaping the molecular world.
Latest Posts
Latest Posts
-
How To Find A Perpendicular Vector
Mar 15, 2025
-
How Could Sulfur Form An Ion
Mar 15, 2025
-
What Elemsnts Are Most Likey To Turn Into Anions Why
Mar 15, 2025
-
What Is The Difference Between Hunger And Appetite
Mar 15, 2025
-
Boiling Point On Graph In Celsius
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about How Many Bonds Can Each Element Have Lewis Structure . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
