How Many Core Electrons Does Xe Have
Muz Play
Mar 15, 2025 · 6 min read
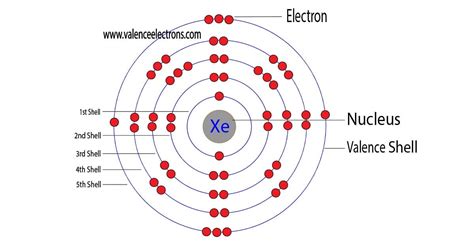
Table of Contents
How Many Core Electrons Does Xe Have? A Deep Dive into Xenon's Electronic Structure
Xenon (Xe), a noble gas residing in Group 18 of the periodic table, is renowned for its chemical inertness. However, understanding its electronic structure, particularly the number of core electrons, unlocks insights into its properties and behavior. This article will delve deep into the electronic configuration of xenon, meticulously explaining how to determine its core electron count, and exploring the implications of this configuration.
Understanding Electronic Configuration
Before diving into xenon's core electrons, it's crucial to grasp the fundamental concept of electronic configuration. This describes the arrangement of electrons within an atom's shells and subshells. Electrons occupy specific energy levels, with those closer to the nucleus having lower energy. These energy levels are designated by principal quantum numbers (n = 1, 2, 3, etc.), and each principal level contains sublevels (s, p, d, f). Each sublevel can accommodate a specific number of electrons: s holds 2, p holds 6, d holds 10, and f holds 14.
The electronic configuration is written as a sequence representing the occupied orbitals and the number of electrons in each. For example, the configuration of oxygen (O) is 1s²2s²2p⁴, indicating two electrons in the 1s orbital, two in the 2s, and four in the 2p.
Determining Xenon's Electronic Configuration
Xenon (Xe) has an atomic number of 54, meaning it possesses 54 electrons. To determine its electronic configuration, we follow the Aufbau principle, filling orbitals in order of increasing energy. The order is: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p, and so on.
Therefore, the complete electronic configuration of xenon is: 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶5s²4d¹⁰5p⁶.
Core Electrons vs. Valence Electrons
Electrons are categorized into two groups: core electrons and valence electrons.
-
Core electrons: These are the electrons found in the inner shells of an atom. They are tightly bound to the nucleus and do not participate directly in chemical bonding. They effectively shield the valence electrons from the full positive charge of the nucleus.
-
Valence electrons: These are the outermost electrons located in the highest energy level (the valence shell). They are loosely held and actively participate in chemical bonding, determining the atom's chemical reactivity.
Identifying Xenon's Core Electrons
To identify xenon's core electrons, we need to examine its electronic configuration and identify the electrons that are not in the outermost shell. The outermost shell for xenon is the fifth shell (n=5), containing the 5s² and 5p⁶ electrons. Therefore, all the electrons in shells 1 to 4 are considered core electrons.
Let's count them:
- Shell 1: 1s² (2 electrons)
- Shell 2: 2s²2p⁶ (8 electrons)
- Shell 3: 3s²3p⁶3d¹⁰ (18 electrons)
- Shell 4: 4s²4p⁶4d¹⁰ (18 electrons)
Adding these up, we find that xenon has a total of 46 core electrons.
The Significance of Core Electrons
While core electrons don't directly participate in bonding, they play several crucial roles:
-
Shielding: Core electrons shield the valence electrons from the full positive charge of the nucleus. This shielding effect reduces the effective nuclear charge experienced by the valence electrons, influencing their energy levels and reactivity. A higher number of core electrons leads to greater shielding.
-
Atomic Size: The number of core electrons significantly contributes to the atom's overall size. More core electrons mean a larger atom, as they occupy more space.
-
Ionization Energy: Core electrons are much more tightly bound to the nucleus than valence electrons. Therefore, they require significantly more energy to remove, leading to higher ionization energies.
-
Chemical Inertness of Noble Gases: The complete octet (or 18-electron configuration in the case of heavier noble gases) in the valence shell of noble gases, coupled with the shielding effect of numerous core electrons, renders these elements exceptionally unreactive. Xenon's high number of core electrons reinforces its chemical inertness, though under specific conditions, it can form compounds.
Xenon's Chemical Behavior and its Core Electrons
The fact that Xenon has 46 core electrons directly impacts its chemical behavior. The large number of core electrons effectively shields the valence electrons from the nucleus, making them less readily available for chemical bonding. This shielding effect is a major contributor to Xenon's famously low reactivity, classifying it as a noble gas.
However, this isn't absolute. While exceptionally unreactive under normal conditions, xenon can form compounds under specific, extreme conditions, such as high pressure or with highly electronegative elements like fluorine and oxygen. Even in these exceptional cases, the influence of the 46 core electrons remains substantial in shaping the properties of the resulting compounds. The strong shielding from the core electrons limits the ability of the nucleus to attract electrons from other atoms strongly, influencing the bonding strength and stability of xenon compounds.
Comparing Xenon to Other Noble Gases
Comparing Xenon to other noble gases provides further perspective on the role of core electrons. Helium (He), for example, has only two electrons, all of which are valence electrons; it has no core electrons. Neon (Ne) has 10 electrons, with 8 valence electrons and 2 core electrons. Argon (Ar) possesses 18 electrons, 8 valence electrons, and 10 core electrons. This gradual increase in the number of core electrons across the noble gases correlates with increased atomic size and slightly reduced ionization energy, reflecting the increasing shielding effect. However, all noble gases exhibit exceptionally low reactivity due to their filled valence shells, even with the varying core electron counts. Xenon's high number of core electrons simply amplifies this already inherent characteristic.
Conclusion: The Crucial Role of Core Electrons in Xenon
In summary, xenon (Xe) possesses 46 core electrons, a significant number contributing substantially to its properties. These core electrons are not directly involved in chemical bonding but play a vital role in shielding the valence electrons, impacting atomic size, ionization energy, and ultimately, xenon's chemical behavior. While xenon’s low reactivity is primarily due to its filled valence shell, the significant shielding provided by its numerous core electrons amplifies this inertness, making it a prime example of the influence of core electron structure on the properties of an element. Understanding the electronic configuration, including the precise count of core electrons, is paramount to comprehending the chemical characteristics and behavior of this unique noble gas. Further research into the behavior of Xenon, under extreme conditions where compound formation does occur, can provide valuable insights into the subtle, yet significant, influence of its extensive core electron structure.
Latest Posts
Latest Posts
-
Energy Required To Remove An Electron From A Gaseous Atom
Mar 15, 2025
-
Que Es La Descomposicion De Acidos
Mar 15, 2025
-
Which Factor Affects Congressional Approval Ratings The Most
Mar 15, 2025
-
Fourier Transform Of A Differential Equation
Mar 15, 2025
-
Which One Neutral Charge Proton Or Neutron
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about How Many Core Electrons Does Xe Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
