How To Calculate Bond Dissociation Energy
Muz Play
Mar 24, 2025 · 6 min read
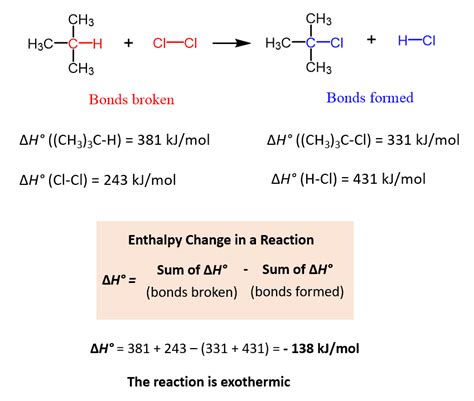
Table of Contents
How to Calculate Bond Dissociation Energy: A Comprehensive Guide
Bond dissociation energy (BDE), also known as bond energy, is a crucial concept in chemistry. It represents the amount of energy required to break a specific chemical bond in a molecule in the gaseous phase. Understanding how to calculate BDE is essential for predicting reaction enthalpies, understanding reaction mechanisms, and gaining insights into molecular stability. This comprehensive guide will delve into the various methods used to determine bond dissociation energies, from experimental techniques to computational approaches.
Understanding Bond Dissociation Energy
Before delving into the calculation methods, let's solidify our understanding of the fundamental concept. Bond dissociation energy specifically refers to the energy change associated with homolytic cleavage of a bond. This means the bond breaks symmetrically, with each atom receiving one electron from the shared pair. This contrasts with heterolytic cleavage, where one atom receives both electrons.
Key Considerations:
- Gaseous Phase: BDE is always measured or calculated for molecules in the gaseous phase. Intermolecular forces in liquids or solids would significantly affect the measured energy.
- Specific Bond: The energy required to break a particular bond varies depending on the molecular environment. For example, the C-H bond energy differs in methane (CH₄), methanol (CH₃OH), and ethane (C₂H₆).
- Average Bond Energies: While we can determine BDE for specific bonds in specific molecules, we often use average bond energies as estimates. These averages are derived from a range of experimental data. However, using averages can introduce inaccuracies, especially for complex molecules.
Experimental Determination of Bond Dissociation Energy
Several experimental techniques directly or indirectly measure bond dissociation energies. The most common are:
1. Spectroscopy
Techniques like photoelectron spectroscopy (PES) and infrared (IR) spectroscopy provide valuable data related to bond strengths. By analyzing the energy of photons required to break a bond or the vibrational frequencies of molecules, we can infer bond dissociation energies. These methods are particularly useful for relatively simple molecules.
2. Calorimetry
Calorimetric methods measure the heat absorbed or released during a chemical reaction. If a reaction involves bond breaking, the heat absorbed can be related to the bond dissociation energy. This approach requires careful control of reaction conditions and precise measurements of heat flow.
3. Kinetic Studies
The rate of a reaction can often be linked to the activation energy, which in turn relates to the bond dissociation energy of the bonds being broken in the rate-determining step. By studying reaction kinetics, researchers can extract information about bond strengths. This method necessitates a thorough understanding of the reaction mechanism.
4. Mass Spectrometry
Mass spectrometry can be used in conjunction with other techniques to analyze the fragments produced when a molecule undergoes bond cleavage. By measuring the mass-to-charge ratio of the fragments and their relative abundances, researchers can gain insights into the bond dissociation energies involved.
Computational Calculation of Bond Dissociation Energy
Computational chemistry offers powerful tools to calculate BDEs, particularly for complex molecules where experimental determination is challenging. These methods typically rely on quantum mechanical calculations.
1. Density Functional Theory (DFT)
DFT is a widely used method that offers a reasonable balance between accuracy and computational cost. DFT calculations can accurately predict BDEs for a wide range of molecules. The choice of functional (the specific mathematical approximation used in DFT) significantly affects the accuracy of the results.
2. Ab Initio Methods
Ab initio methods, such as coupled cluster (CC) and Møller-Plesset perturbation theory (MP2), offer higher accuracy than DFT but are significantly more computationally expensive. They are often used for smaller molecules or to validate DFT results. These methods solve the Schrödinger equation directly or through approximations that aim to get as close as possible to an exact solution.
3. Selecting the Right Computational Method
The optimal computational approach depends on factors like molecular size, desired accuracy, and available computational resources. For large molecules, DFT is often the only feasible option. For smaller molecules, high-level ab initio methods can provide higher accuracy but at a significant cost in computational time and resources.
Important Considerations in Computational Calculations:
- Basis Set: The choice of basis set, which defines the mathematical functions used to represent the molecular orbitals, influences the accuracy of the calculation. Larger basis sets generally lead to more accurate results but require more computational resources.
- Level of Theory: The level of theory refers to the specific computational method employed (e.g., DFT with B3LYP functional, MP2). The level of theory directly affects the accuracy and computational cost.
- Optimization: Before calculating the bond dissociation energy, the molecular geometries of both the reactant (molecule with the intact bond) and the products (fragments after bond breakage) must be optimized to ensure they are at their lowest energy state.
Calculating BDE from Enthalpy Changes
Bond dissociation energies can be inferred from experimentally determined enthalpy changes (ΔH) of reactions. If the reaction involves the breaking of a specific bond, the enthalpy change is directly related to the bond dissociation energy.
Example:
Consider the gas-phase reaction:
CH₄(g) → CH₃(g) + H(g)
The enthalpy change (ΔH) for this reaction corresponds to the bond dissociation energy of the C-H bond in methane. If ΔH is experimentally determined to be +439 kJ/mol, then the BDE of the C-H bond in methane is 439 kJ/mol. This is because the reaction is endothermic; energy must be input to break the bond.
Interpreting and Applying Bond Dissociation Energies
BDE values provide crucial information about:
- Reaction Feasibility: Reactions involving the breaking of strong bonds typically require significant energy input and may be less favorable.
- Reaction Mechanisms: Knowing the BDEs of bonds involved in a reaction helps predict which bonds are likely to break and in what order, providing insights into the reaction mechanism.
- Molecular Stability: Molecules with stronger bonds are generally more stable.
- Predicting Reaction Enthalpies: BDEs are fundamental in calculating the enthalpy changes (ΔH) for many chemical reactions, particularly those involving bond breaking and formation.
Advanced Concepts and Considerations
- Multiple Bonds: Calculating BDEs for multiple bonds (double bonds, triple bonds) requires considering the sequential breaking of each component of the multiple bond.
- Radical Stability: The stability of the resulting radicals after bond cleavage significantly impacts the BDE. Stabilizing factors, like resonance or inductive effects, can lower the BDE.
- Temperature Dependence: BDEs can show a slight temperature dependence, although this effect is often small.
- Solvent Effects: While BDEs are defined for the gas phase, solvent effects can significantly affect bond strengths in solution.
Conclusion
Calculating bond dissociation energy is a fundamental task in chemistry with implications spanning reaction prediction, mechanism elucidation, and the understanding of molecular stability. Whether using experimental or computational techniques, accurate determination of BDE requires careful consideration of methodological details and potential sources of error. The insights gained from these calculations are critical for advancing our understanding of chemical reactivity and molecular behavior. This comprehensive guide has equipped you with the knowledge to understand and apply various methods for calculating bond dissociation energies, allowing you to interpret and utilize these crucial values in your chemical endeavors. Remember to consult specialized literature and resources for advanced applications and more nuanced considerations.
Latest Posts
Latest Posts
-
Chemical Equilibrium Le Chateliers Principle Lab Answers
Mar 26, 2025
-
Peroxisomes Got Their Name Because Hydrogen Peroxide Is
Mar 26, 2025
-
State Of Matter With Definite Shape And Volume
Mar 26, 2025
-
Question Lexan Draw The Monomer Used To Make This Polymer
Mar 26, 2025
-
How Do You Calculate Index Numbers
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about How To Calculate Bond Dissociation Energy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
