How To Find Molar Mass Of Gas
Muz Play
Mar 22, 2025 · 6 min read
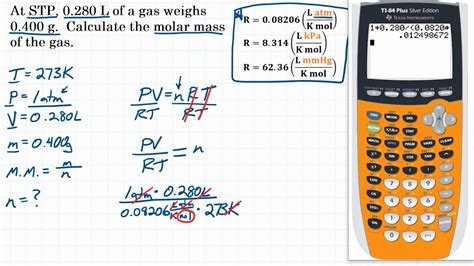
Table of Contents
How to Find the Molar Mass of a Gas: A Comprehensive Guide
Determining the molar mass of a gas is a fundamental concept in chemistry with applications spanning various fields. From environmental monitoring to industrial process control, understanding how to calculate this crucial value is essential. This comprehensive guide will walk you through various methods, providing a detailed understanding of the principles involved and equipping you with the tools to tackle diverse problems.
Understanding Molar Mass
Before diving into the methods, let's establish a clear definition. Molar mass is the mass of one mole of a substance. A mole is a unit representing Avogadro's number (approximately 6.022 x 10<sup>23</sup>) of particles, whether atoms, molecules, or ions. The molar mass is typically expressed in grams per mole (g/mol). For gases, determining the molar mass allows us to identify the gas and understand its behavior under different conditions.
Methods for Determining Molar Mass of a Gas
Several methods exist for finding the molar mass of a gas, each relying on different principles and experimental setups. We will explore the most common and practical approaches.
1. Using the Ideal Gas Law
The Ideal Gas Law, PV = nRT, is a cornerstone of gas-phase chemistry. This equation relates pressure (P), volume (V), number of moles (n), gas constant (R), and temperature (T). By manipulating this equation, we can derive a formula for molar mass (M).
-
Derivation:
We know that the number of moles (n) can be expressed as the mass (m) divided by the molar mass (M): n = m/M. Substituting this into the Ideal Gas Law, we get:
PV = (m/M)RT
Solving for Molar Mass (M):
M = mRT/PV
-
Variables:
- m: Mass of the gas (in grams)
- R: Ideal gas constant (0.0821 L·atm/mol·K or other appropriate units depending on the units of other variables)
- T: Temperature (in Kelvin)
- P: Pressure (in atmospheres, Pascals, etc., consistent with R)
- V: Volume (in liters, cubic meters, etc., consistent with R)
-
Example:
Let's say we have 2.00 g of an unknown gas occupying 1.50 L at 25°C and 1.00 atm. To find its molar mass:
- Convert temperature to Kelvin: T = 25°C + 273.15 = 298.15 K
- Substitute the values into the formula: M = (2.00 g)(0.0821 L·atm/mol·K)(298.15 K) / (1.00 atm)(1.50 L)
- Calculate: M ≈ 32.7 g/mol
This method assumes ideal gas behavior, which is a good approximation for many gases at moderate temperatures and pressures. However, at high pressures or low temperatures, deviations from ideality may occur, requiring corrections or the use of more sophisticated equations of state.
2. Using Density
The density (ρ) of a gas is its mass (m) per unit volume (V): ρ = m/V. We can combine this with the Ideal Gas Law to derive another equation for molar mass.
-
Derivation:
Starting with the Ideal Gas Law (PV = nRT) and substituting n = m/M, we get:
PV = (m/M)RT
Rearranging for M:
M = mRT/PV
Since ρ = m/V, we can substitute this into the equation:
M = ρRT/P
-
Variables:
- ρ: Density of the gas (in g/L or other consistent units)
- R: Ideal gas constant (choose the appropriate units based on the density units)
- T: Temperature (in Kelvin)
- P: Pressure (in atmospheres, Pascals, etc., consistent with R and ρ)
-
Example:
If the density of a gas is 1.96 g/L at 273.15 K and 1 atm, then:
M = (1.96 g/L)(0.0821 L·atm/mol·K)(273.15 K) / (1 atm) ≈ 44 g/mol
3. Using Effusion or Diffusion Rates (Graham's Law)
Graham's Law states that the rate of effusion or diffusion of a gas is inversely proportional to the square root of its molar mass. This provides another method for comparing the molar masses of two gases.
-
Graham's Law Equation:
Rate<sub>1</sub> / Rate<sub>2</sub> = √(M<sub>2</sub> / M<sub>1</sub>)
Where:
- Rate<sub>1</sub> and Rate<sub>2</sub> are the rates of effusion or diffusion of gas 1 and gas 2, respectively.
- M<sub>1</sub> and M<sub>2</sub> are the molar masses of gas 1 and gas 2, respectively.
-
Application:
This method is particularly useful when comparing the molar mass of an unknown gas to a known gas. By measuring the relative rates of effusion or diffusion (e.g., time taken for a certain volume to effuse through a small hole), we can determine the unknown molar mass.
-
Example:
If gas A effuses twice as fast as gas B, and the molar mass of gas B is 32 g/mol, then:
2 = √(32 g/mol / M<sub>A</sub>)
Solving for M<sub>A</sub>: M<sub>A</sub> = 8 g/mol
4. Mass Spectrometry
Mass spectrometry is a sophisticated analytical technique that provides highly accurate molar mass measurements. It involves ionizing gas molecules and separating them based on their mass-to-charge ratio. The resulting mass spectrum shows the relative abundance of different ions, allowing for precise determination of the molar mass. This technique is very accurate but requires specialized equipment.
Factors Affecting Accuracy
Several factors can influence the accuracy of molar mass determination.
-
Non-ideal Gas Behavior: At high pressures or low temperatures, gases deviate from ideal behavior. Using the ideal gas law may lead to significant errors in these conditions. More complex equations of state, such as the van der Waals equation, may be necessary for accurate calculations.
-
Experimental Errors: Errors in measuring pressure, volume, temperature, and mass can all affect the accuracy of the results. Careful experimental technique and precise measurement tools are essential.
-
Gas Purity: The presence of impurities in the gas sample can lead to inaccurate molar mass determination. Purification of the gas sample is crucial for reliable results.
-
Incomplete Reactions: If the molar mass is determined through a chemical reaction, incomplete reactions can lead to errors. Ensure the reaction is complete before measurements are made.
Choosing the Right Method
The optimal method for determining the molar mass of a gas depends on several factors, including the available equipment, the accuracy required, and the properties of the gas.
-
For routine measurements with readily available equipment, using the ideal gas law or density is often sufficient.
-
For higher accuracy, mass spectrometry is the preferred method.
-
If comparing the molar mass of an unknown gas to a known gas, Graham's Law can be a useful technique.
Conclusion
Determining the molar mass of a gas is a vital skill in chemistry with wide-ranging applications. Understanding the underlying principles and selecting the appropriate method based on the situation are essential for accurate and reliable results. This guide provides a solid foundation for tackling various problems related to gas molar mass determination, allowing you to effectively utilize this fundamental concept in your studies and research. Remember always to double-check your units and ensure consistency throughout your calculations to minimize errors and achieve the most accurate results.
Latest Posts
Latest Posts
-
Does Electric Potential Increase With Distance
Mar 23, 2025
-
How To Calculate Binding Energy Per Nucleon
Mar 23, 2025
-
What Are The Products Of This Chemical Reaction
Mar 23, 2025
-
A Neutral Atom Has Equal Numbers Of Blank And Electrons
Mar 23, 2025
-
Environmental Factors That Affect Microbial Growth
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about How To Find Molar Mass Of Gas . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
