How To Know If A Structure Is Polar Or Nonpolar
Muz Play
Apr 01, 2025 · 6 min read
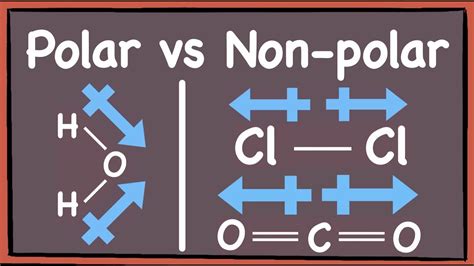
Table of Contents
How to Know if a Structure is Polar or Nonpolar: A Comprehensive Guide
Determining whether a molecule is polar or nonpolar is crucial in understanding its properties and behavior. Polarity significantly impacts physical properties like boiling point, melting point, solubility, and reactivity. This comprehensive guide will equip you with the knowledge and tools to confidently identify the polarity of various molecular structures.
Understanding Polarity: The Basics
Polarity arises from the unequal sharing of electrons in a covalent bond. This unequal sharing is caused by a difference in electronegativity between the atoms involved. Electronegativity is a measure of an atom's ability to attract electrons towards itself within a chemical bond.
-
Nonpolar molecules: In nonpolar molecules, electrons are shared relatively equally between atoms. This typically occurs when the atoms have similar electronegativities or when the molecule is symmetrical, canceling out any individual bond dipoles.
-
Polar molecules: In polar molecules, electrons are shared unequally, resulting in a dipole moment. A dipole moment is a vector quantity that represents the separation of positive and negative charges within a molecule. It's represented by an arrow pointing from the positive end (δ+) to the negative end (δ-). The greater the difference in electronegativity, the larger the dipole moment, and the more polar the molecule.
Determining Molecular Polarity: A Step-by-Step Approach
Identifying the polarity of a molecule involves a systematic approach:
1. Draw the Lewis Structure
The first step is to accurately draw the Lewis structure of the molecule. This visually represents the arrangement of atoms and bonding electrons. It's essential to identify all single, double, and triple bonds and any lone pairs of electrons. Accurate Lewis structures are crucial for determining molecular geometry.
2. Determine the Molecular Geometry
Molecular geometry describes the three-dimensional arrangement of atoms in a molecule. This is crucial because the overall polarity of a molecule depends not only on individual bond dipoles but also on their spatial arrangement. Common molecular geometries include:
- Linear: Atoms arranged in a straight line (e.g., CO<sub>2</sub>).
- Bent: Atoms arranged in a V-shape (e.g., H<sub>2</sub>O).
- Trigonal Planar: Atoms arranged in a flat triangle (e.g., BF<sub>3</sub>).
- Tetrahedral: Atoms arranged in a pyramid with four triangular faces (e.g., CH<sub>4</sub>).
- Trigonal Pyramidal: Atoms arranged in a pyramid with three triangular faces (e.g., NH<sub>3</sub>).
- Octahedral: Atoms arranged in a symmetrical octahedron (e.g., SF<sub>6</sub>).
You can use VSEPR (Valence Shell Electron Pair Repulsion) theory to predict the molecular geometry based on the number of bonding and non-bonding electron pairs around the central atom.
3. Identify Bond Polarity
Examine each individual bond within the molecule. Use a periodic table to compare the electronegativities of the atoms involved in each bond. If there's a significant difference in electronegativity (typically greater than 0.4), the bond is considered polar. The more electronegative atom will carry a partial negative charge (δ-), while the less electronegative atom will carry a partial positive charge (δ+).
4. Determine the Overall Molecular Polarity
This is the most crucial step. Even if individual bonds are polar, the molecule as a whole might be nonpolar due to symmetry. Here's how to determine the overall polarity:
-
Symmetrical Molecules: If the individual bond dipoles cancel each other out due to symmetry, the molecule is nonpolar. For instance, in CO<sub>2</sub>, the two C=O bonds are polar, but they are oriented in opposite directions, resulting in a net dipole moment of zero. Examples include linear molecules with identical atoms bonded to the central atom (like CO<sub>2</sub>) and tetrahedral molecules with identical atoms surrounding the central atom (like CH<sub>4</sub>).
-
Asymmetrical Molecules: If the individual bond dipoles do not cancel each other out due to asymmetry in the molecular geometry, the molecule is polar. The net dipole moment is the vector sum of the individual bond dipoles. Water (H<sub>2</sub>O) is a classic example. The O-H bonds are polar, and the bent geometry prevents the bond dipoles from canceling each other, resulting in a net dipole moment and a polar molecule. Ammonia (NH<sub>3</sub>) is another example.
Examples: Putting it all together
Let's apply this step-by-step approach to some examples:
Example 1: Carbon Dioxide (CO<sub>2</sub>)
- Lewis Structure: O=C=O
- Molecular Geometry: Linear
- Bond Polarity: C=O bonds are polar (oxygen is more electronegative than carbon).
- Overall Polarity: Nonpolar. The two C=O bond dipoles are equal in magnitude but opposite in direction, resulting in a net dipole moment of zero.
Example 2: Water (H<sub>2</sub>O)
- Lewis Structure: H-O-H (with two lone pairs on oxygen)
- Molecular Geometry: Bent
- Bond Polarity: O-H bonds are polar (oxygen is more electronegative than hydrogen).
- Overall Polarity: Polar. The bent geometry prevents the O-H bond dipoles from canceling each other out, resulting in a net dipole moment.
Example 3: Methane (CH<sub>4</sub>)
- Lewis Structure: A central carbon atom bonded to four hydrogen atoms.
- Molecular Geometry: Tetrahedral
- Bond Polarity: C-H bonds are slightly polar (carbon is slightly more electronegative than hydrogen).
- Overall Polarity: Nonpolar. The symmetrical tetrahedral arrangement ensures that the individual bond dipoles cancel each other out completely.
Example 4: Ammonia (NH<sub>3</sub>)
- Lewis Structure: A central nitrogen atom bonded to three hydrogen atoms, with one lone pair of electrons on nitrogen.
- Molecular Geometry: Trigonal Pyramidal
- Bond Polarity: N-H bonds are polar (nitrogen is more electronegative than hydrogen).
- Overall Polarity: Polar. The trigonal pyramidal geometry does not allow for the cancellation of the bond dipoles, resulting in a net dipole moment.
Advanced Considerations: Factors influencing polarity
Several factors can influence the polarity of molecules beyond the basics outlined above:
- Resonance: In molecules with resonance structures, the delocalization of electrons can affect the overall dipole moment.
- Inductive Effects: The presence of electronegative or electropositive substituents can influence the electron density distribution and, consequently, the polarity of the molecule.
- Hydrogen Bonding: Hydrogen bonding, a strong type of intermolecular force, is a consequence of the polarity of molecules containing O-H, N-H, or F-H bonds. It significantly influences the physical properties of such molecules.
Conclusion: Mastering Molecular Polarity
Understanding the polarity of molecules is fundamental to comprehending their properties and behavior. By systematically following the steps outlined in this guide—drawing the Lewis structure, determining the molecular geometry, identifying bond polarities, and assessing the overall molecular symmetry—you can confidently determine whether a molecule is polar or nonpolar. Remember that while the basics provide a strong foundation, advanced concepts like resonance and inductive effects can add complexity to the analysis in some cases. Mastering these concepts will significantly enhance your understanding of chemistry and its applications.
Latest Posts
Latest Posts
-
Is Magnesium A Metal Nonmetal Or Metalloid
Apr 02, 2025
-
Number Of Atoms In A Simple Cubic Unit Cell
Apr 02, 2025
-
Inscribed Circle In A Right Triangle
Apr 02, 2025
-
Bacteria And Archaea Are Both Domains Consisting Of Prokaryotic Organisms
Apr 02, 2025
-
What Organelles Do Plants Have That Animals Dont
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about How To Know If A Structure Is Polar Or Nonpolar . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
