How To Read H Nmr Spectrum
Muz Play
Mar 20, 2025 · 7 min read
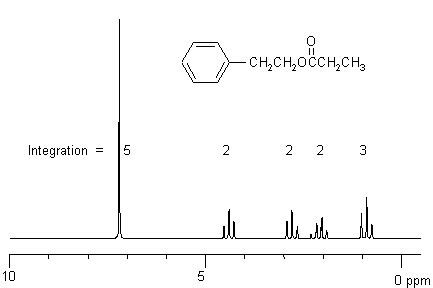
Table of Contents
How to Read an H NMR Spectrum: A Comprehensive Guide
Nuclear Magnetic Resonance (NMR) spectroscopy, specifically proton NMR (¹H NMR), is a powerful analytical technique used extensively in chemistry to determine the structure of organic molecules. Understanding how to interpret an ¹H NMR spectrum is crucial for chemists, students, and anyone working with organic compounds. This comprehensive guide will walk you through the fundamental principles and provide practical steps to decipher the information encoded within an ¹H NMR spectrum.
Understanding the Basics: Key Concepts in ¹H NMR
Before diving into the interpretation, it's essential to grasp the fundamental concepts underpinning ¹H NMR.
The Principle of Nuclear Spin:
The foundation of NMR lies in the property of nuclear spin. Many atomic nuclei possess an intrinsic angular momentum, or spin, which can be visualized as a tiny spinning magnet. Hydrogen's nucleus (¹H) has a spin of ½, meaning it can exist in two spin states: +½ and -½. In the absence of an external magnetic field, these spin states are degenerate (have the same energy).
The Effect of an External Magnetic Field:
When a sample containing ¹H nuclei is placed in a strong external magnetic field (B₀), the degeneracy of the spin states is lifted. The nuclei align either parallel (lower energy, α-spin) or antiparallel (higher energy, β-spin) to the applied magnetic field. This energy difference is crucial for NMR spectroscopy.
Resonance and Radiofrequency Absorption:
Radiofrequency (RF) radiation is then applied to the sample. If the energy of the RF radiation exactly matches the energy difference between the α and β spin states, the nuclei will absorb the radiation and undergo a transition from the lower energy (α) to the higher energy (β) state. This absorption of energy is detected and forms the basis of the NMR spectrum. The frequency at which this absorption occurs is known as the resonance frequency and depends on the strength of the magnetic field and the chemical environment of the nucleus.
Chemical Shift:
The resonance frequency of a proton is not solely determined by the applied magnetic field. The electrons surrounding the proton also generate their own small magnetic fields, which shield the proton from the external magnetic field. This shielding effect varies depending on the chemical environment of the proton, leading to different resonance frequencies for protons in different parts of a molecule. The difference in resonance frequency relative to a standard (usually tetramethylsilane, TMS) is called the chemical shift (δ), measured in parts per million (ppm). Different functional groups give rise to characteristic chemical shifts.
Deciphering the ¹H NMR Spectrum: A Step-by-Step Guide
A typical ¹H NMR spectrum consists of a plot of chemical shift (δ, in ppm) on the x-axis and signal intensity (related to the number of protons) on the y-axis. Each peak represents a group of chemically equivalent protons.
1. Identifying the Number of Signals:
The first step in interpreting a ¹H NMR spectrum is to count the number of distinct signals. Each signal represents a set of chemically equivalent protons. Chemically equivalent protons are protons that have the same chemical environment and thus experience the same shielding effect.
2. Determining the Integration Values:
The area under each signal is proportional to the number of protons giving rise to that signal. The NMR spectrometer usually provides integration values, which are represented as a ratio of the number of protons. For example, a ratio of 3:2 indicates that one signal corresponds to three protons and the other to two protons. These integration values are essential for determining the relative number of protons in each chemical environment.
3. Analyzing Chemical Shifts (δ):
The chemical shift (δ) provides crucial information about the electronic environment of the protons. Different functional groups exhibit characteristic chemical shift ranges. Knowing these ranges is vital for assigning peaks to specific protons in the molecule.
- 0-2 ppm: Protons attached to sp³ hybridized carbons, typically alkyl groups (CH₃, CH₂, CH).
- 2-3 ppm: Protons on carbons adjacent to heteroatoms (e.g., -CH₂-O-, -CH₂-N-).
- 3.5-4.5 ppm: Protons attached to sp³ hybridized carbons directly bonded to electronegative atoms (e.g., -CH₂-Cl, -CH₂-OH).
- 4.5-6.5 ppm: Protons attached to sp² hybridized carbons (e.g., alkenes, aromatic rings).
- 6.5-8 ppm: Protons in aromatic rings.
- 9-13 ppm: Protons attached to carbonyl groups (e.g., -COOH, -CHO).
It's crucial to note that these ranges are approximate, and the exact chemical shift can be influenced by neighboring groups and other factors.
4. Interpreting Splitting Patterns (Multiplicity):
The splitting pattern (multiplicity) of each signal provides information about the number of neighboring protons. This splitting is governed by the n+1 rule, where n is the number of equivalent neighboring protons.
- Singlet (s): No neighboring protons (n = 0).
- Doublet (d): One neighboring proton (n = 1).
- Triplet (t): Two neighboring protons (n = 2).
- Quartet (q): Three neighboring protons (n = 3).
- Multiplet (m): More than three neighboring protons, or overlapping signals.
The n+1 rule applies only when the chemical shift difference between the coupled protons is significantly greater than their coupling constant (J). If the chemical shift difference is smaller, more complex splitting patterns may be observed.
5. Coupling Constants (J):
The coupling constant (J) is a measure of the strength of the coupling between neighboring protons. It is expressed in Hertz (Hz) and is usually measured as the distance between adjacent peaks within a multiplet. The value of J provides information about the geometry of the molecule and the type of coupling (e.g., geminal, vicinal).
6. Putting it All Together: Structural Elucidation:
By systematically analyzing the number of signals, integration values, chemical shifts, splitting patterns, and coupling constants, one can piece together the structure of the molecule. This often involves a process of elimination and considering possible structures consistent with the spectral data. Referencing chemical shift tables and utilizing spectral databases can also be incredibly helpful.
Advanced ¹H NMR Concepts
Spin-Spin Coupling:
Spin-spin coupling is the interaction between the magnetic moments of neighboring nuclei through covalent bonds. This interaction leads to the splitting of signals, as described by the n+1 rule. Understanding different types of coupling, like geminal (between protons on the same carbon) and vicinal (between protons on adjacent carbons), is crucial for complex structure elucidation.
Exchange Processes:
Protons involved in rapid exchange processes, such as those in -OH or -NH groups, may not show typical splitting patterns. The exchange rate can be influenced by factors such as solvent, temperature, and the presence of catalysts.
Solvent Effects:
The choice of solvent can affect both the chemical shift and the splitting patterns observed in an ¹H NMR spectrum. Solvent molecules can interact with the analyte molecules, leading to changes in the shielding effects and hence the chemical shifts.
Dynamic NMR:
Dynamic NMR studies the effects of molecular motions on NMR spectra. By varying the temperature or using other experimental parameters, one can study the kinetics and thermodynamics of conformational changes or other dynamic processes in molecules.
2D NMR Techniques:
While ¹H NMR provides valuable structural information, two-dimensional (2D) NMR techniques offer enhanced resolution and allow for the identification of long-range couplings and correlations between different protons in complex molecules. Common 2D NMR techniques include COSY (Correlation Spectroscopy) and NOESY (Nuclear Overhauser Effect Spectroscopy).
Practical Tips for Reading ¹H NMR Spectra
- Start with the basics: Begin by analyzing the number of signals, integration values, and chemical shifts.
- Use chemical shift tables: Refer to comprehensive chemical shift tables to aid in assigning peaks to specific functional groups.
- Consider splitting patterns: Carefully analyze the multiplicity of each signal to infer the number of neighboring protons.
- Pay attention to coupling constants: The values of coupling constants provide important information about the geometry of the molecule.
- Use online resources: Several online databases and spectral analysis tools can be used to aid in the interpretation of NMR spectra.
- Practice, practice, practice: The most effective way to become proficient in interpreting NMR spectra is to practice regularly. Start with simple spectra and gradually work your way up to more complex ones.
By systematically applying these steps and concepts, one can successfully interpret ¹H NMR spectra and extract crucial structural information about organic molecules. Remember that the interpretation of NMR spectra is a skill that develops with experience and consistent practice. With patience and dedication, you can master this powerful analytical technique and unlock the secrets hidden within the complex world of organic molecules.
Latest Posts
Latest Posts
-
Is Carbon Dioxide A Pure Substance
Mar 21, 2025
-
Map Of North Africa Southwest Asia
Mar 21, 2025
-
What Is The Function Of Stem
Mar 21, 2025
-
What Is The Property Of A Base
Mar 21, 2025
-
How To Find The Excess Reactant
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about How To Read H Nmr Spectrum . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
