How To Tell If A Reaction Is Redox
Muz Play
Mar 21, 2025 · 6 min read
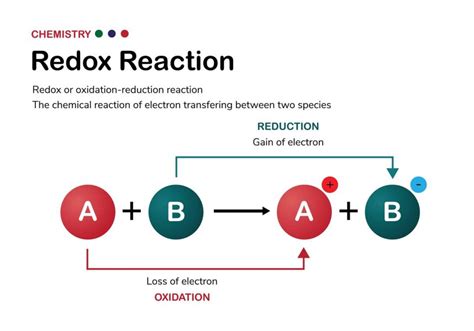
Table of Contents
How to Tell if a Reaction is Redox: A Comprehensive Guide
Redox reactions, short for reduction-oxidation reactions, are fundamental processes in chemistry and biology. They involve the transfer of electrons between species, leading to changes in oxidation states. Understanding how to identify a redox reaction is crucial for comprehending a wide array of phenomena, from combustion to photosynthesis and even corrosion. This comprehensive guide will equip you with the knowledge and tools to confidently determine whether a chemical reaction is a redox reaction.
Understanding Oxidation and Reduction
Before diving into identifying redox reactions, let's solidify our understanding of the core concepts: oxidation and reduction. These processes are always coupled; one cannot occur without the other.
Oxidation: Loss of Electrons
Oxidation is defined as the loss of electrons by a species. This loss results in an increase in the oxidation state of the atom involved. Think of it as the atom becoming more positive, either by losing negatively charged electrons or gaining a more electronegative atom.
Key Indicators of Oxidation:
- Increase in oxidation state: This is the most reliable indicator. We'll discuss how to calculate oxidation states later.
- Loss of hydrogen atoms: Often, hydrogen atoms are involved in redox reactions. Losing hydrogen is a sign of oxidation.
- Gain of oxygen atoms: Similarly, gaining oxygen atoms frequently indicates oxidation. This is why reactions with oxygen are often called oxidation reactions.
Reduction: Gain of Electrons
Reduction is the opposite of oxidation; it's the gain of electrons by a species. This gain results in a decrease in the oxidation state of the atom involved. The atom becomes less positive (more negative).
Key Indicators of Reduction:
- Decrease in oxidation state: Again, the change in oxidation state is paramount.
- Gain of hydrogen atoms: Adding hydrogen atoms signifies reduction.
- Loss of oxygen atoms: Losing oxygen atoms is a typical sign of reduction.
Assigning Oxidation States: The Key to Identifying Redox Reactions
Accurately determining whether a reaction is redox hinges on correctly assigning oxidation states (also known as oxidation numbers). Here's a step-by-step guide:
-
Free elements: The oxidation state of an atom in its elemental form is always zero. For example, the oxidation state of O₂ is 0, and the oxidation state of Fe is 0.
-
Monatomic ions: The oxidation state of a monatomic ion is equal to its charge. For example, the oxidation state of Na⁺ is +1, and the oxidation state of Cl⁻ is -1.
-
Hydrogen: Hydrogen typically has an oxidation state of +1, except when bonded to metals in binary compounds (such as metal hydrides, where it's -1).
-
Oxygen: Oxygen usually has an oxidation state of -2, except in peroxides (where it's -1) and in compounds with fluorine (where it can be positive).
-
Fluorine: Fluorine always has an oxidation state of -1.
-
Other elements: For other elements, the oxidation state is assigned to make the sum of oxidation states in a neutral molecule equal to zero, and in a polyatomic ion, equal to the charge of the ion. This often requires some algebraic manipulation.
Example: Let's determine the oxidation states in H₂SO₄.
- Hydrogen (H): +1 (two H atoms, total +2)
- Oxygen (O): -2 (four O atoms, total -8)
- Sulfur (S): To balance the charges, the sulfur must have an oxidation state of +6 (+2 + (+6) + (-8) = 0).
Identifying Redox Reactions: A Practical Approach
Now that we can assign oxidation states, we can confidently identify redox reactions. A reaction is redox if at least one element undergoes a change in oxidation state. If no change in oxidation states occurs, it's not a redox reaction.
Step-by-step procedure:
-
Assign oxidation states to all atoms in both the reactants and products.
-
Compare the oxidation states of each atom in the reactants to its oxidation state in the products.
-
If any atom's oxidation state changes, the reaction is a redox reaction. If no oxidation states change, it's not a redox reaction.
Example 1: A Redox Reaction
Consider the reaction: 2Mg(s) + O₂(g) → 2MgO(s)
- Reactants: Mg(s) (oxidation state: 0), O₂(g) (oxidation state: 0)
- Products: MgO(s) (Mg: +2, O: -2)
Magnesium goes from an oxidation state of 0 to +2 (oxidation), while oxygen goes from 0 to -2 (reduction). Since there's a change in oxidation states, this is a redox reaction.
Example 2: Not a Redox Reaction
Consider the reaction: HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l)
- Reactants: H⁺(+1), Cl⁻(-1), Na⁺(+1), OH⁻(-1)
- Products: Na⁺(+1), Cl⁻(-1), H⁺(+1), O⁻²(-2)
The oxidation states of all atoms remain the same throughout the reaction. Therefore, this is not a redox reaction; it's an acid-base neutralization reaction.
Recognizing Redox Reactions Through Other Clues
While the change in oxidation states is the definitive test, certain reaction patterns often hint at a redox reaction:
- Reactions involving combustion: Combustion reactions, where a substance reacts rapidly with oxygen, are almost always redox reactions.
- Reactions involving single displacement: Single displacement reactions (also called single replacement reactions), where one element replaces another in a compound, are typically redox reactions.
- Reactions involving disproportionation: Disproportionation reactions involve a single species undergoing both oxidation and reduction simultaneously.
- Reactions involving the transfer of electrons (explicitly shown): If a reaction explicitly depicts the transfer of electrons (e.g., using e⁻ notation), it's definitely a redox reaction.
- Reactions involving strong oxidizing or reducing agents: The presence of strong oxidizing agents (like permanganate, dichromate, or hydrogen peroxide) or reducing agents (like lithium aluminum hydride or sodium borohydride) strongly suggests a redox reaction.
Complex Redox Reactions and Balancing
Some redox reactions can be quite complex, involving many reactants and products. Balancing these equations can be challenging. However, methods exist, like the half-reaction method, which simplifies the balancing process by separating the overall reaction into two half-reactions: one for oxidation and one for reduction. This method ensures that the number of electrons lost in oxidation equals the number of electrons gained in reduction.
Applications of Redox Reactions
Redox reactions are ubiquitous in our world and play vital roles in numerous processes:
- Corrosion: The rusting of iron is a classic example of a redox reaction, where iron is oxidized by oxygen in the presence of water.
- Batteries: Batteries utilize redox reactions to generate electrical energy. The transfer of electrons drives the flow of current.
- Photosynthesis: This crucial biological process involves a series of redox reactions, where water is oxidized and carbon dioxide is reduced to form glucose.
- Respiration: Cellular respiration, the process by which organisms obtain energy from food, also involves redox reactions.
- Metallurgy: The extraction of metals from their ores frequently involves redox reactions.
Conclusion
Identifying whether a reaction is a redox reaction is a crucial skill in chemistry. By mastering the techniques of assigning oxidation states and understanding the principles of oxidation and reduction, you can confidently analyze chemical reactions and understand the fundamental processes at play. Remember, the change in oxidation states is the definitive indicator, but recognizing common patterns and reaction types can provide valuable clues. Understanding redox reactions unlocks a deeper understanding of many fundamental processes in nature and technology.
Latest Posts
Latest Posts
-
What Does Newtons First Law Say
Mar 28, 2025
-
Does Ionic Have High Boiling Point
Mar 28, 2025
-
Is Dipole Dipole Polar Or Nonpolar
Mar 28, 2025
-
A Hydrocarbon Is Any Compound That Contains
Mar 28, 2025
-
In Order To Break A Bond Energy Must Be
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about How To Tell If A Reaction Is Redox . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
