In A Lewis Formula The Dots Represent
Muz Play
Mar 15, 2025 · 6 min read
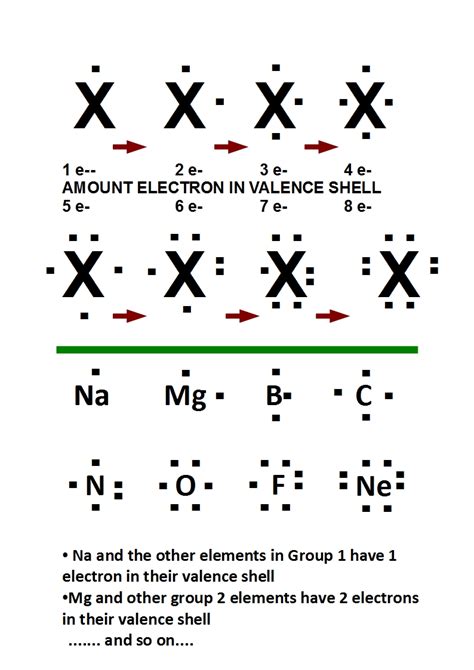
Table of Contents
In a Lewis Formula, the Dots Represent: A Deep Dive into Electron Dot Structures
Lewis formulas, also known as Lewis dot structures or electron dot diagrams, are essential tools in chemistry for visualizing the valence electrons of atoms and molecules. Understanding what the dots represent in these diagrams is fundamental to grasping chemical bonding, molecular geometry, and predicting the properties of substances. This comprehensive guide will explore the meaning of dots in Lewis formulas, their application in different bonding scenarios, and advanced considerations for complex molecules.
Understanding the Basics: Valence Electrons and the Octet Rule
Before delving into the specifics of Lewis formulas, let's establish a firm understanding of the core concepts. The dots in a Lewis formula represent valence electrons. These are the electrons located in the outermost shell (valence shell) of an atom. Valence electrons are crucial because they are the electrons involved in chemical bonding.
The octet rule, a fundamental principle in chemistry, states that atoms tend to gain, lose, or share electrons in order to achieve a stable electron configuration with eight electrons in their valence shell. This configuration mimics the stable electron configuration of noble gases, which are exceptionally unreactive. While there are exceptions to the octet rule (e.g., elements like boron and phosphorus can have fewer than eight valence electrons), it provides a useful framework for understanding bonding in a vast majority of molecules.
How Dots Depict Valence Electrons in Lewis Formulas
The dots in a Lewis formula are strategically placed around the element's symbol to represent the valence electrons. Each dot represents a single valence electron. For example:
- Hydrogen (H): Hydrogen has one valence electron, so its Lewis dot structure is simply: •H
- Oxygen (O): Oxygen has six valence electrons, and its Lewis dot structure is typically represented as: :Ö: (where the pairs of dots represent electron pairs)
- Carbon (C): Carbon has four valence electrons, depicted as: •C•
The arrangement of dots aims to visually represent the distribution of these valence electrons. Single dots represent unpaired electrons, while pairs of dots represent paired electrons. This visual representation helps us understand how atoms will interact to form chemical bonds.
Lewis Structures and Chemical Bonding: Ionic, Covalent, and Coordinate Bonds
The Lewis dot structures are instrumental in illustrating the different types of chemical bonds:
1. Ionic Bonds: Transfer of Electrons
Ionic bonds form when there's a significant difference in electronegativity between two atoms. One atom (typically a metal) loses one or more valence electrons, becoming a positively charged ion (cation), while another atom (typically a non-metal) gains these electrons, becoming a negatively charged ion (anion). The electrostatic attraction between these oppositely charged ions constitutes the ionic bond. Lewis structures show this electron transfer by depicting the ions with their corresponding charges. For example, in the formation of NaCl (sodium chloride), sodium (Na) loses one electron to chlorine (Cl):
Na• + :Cl• → Na⁺ + :Cl:⁻
2. Covalent Bonds: Sharing of Electrons
Covalent bonds form when atoms share valence electrons to achieve a stable octet. This sharing is represented in Lewis structures by lines connecting the atoms. Each line represents a shared pair of electrons (a single covalent bond). Multiple bonds (double or triple bonds) are possible when atoms share two or three pairs of electrons, respectively. For example:
- Water (H₂O): H-O-H (Each dash represents two electrons, one from each hydrogen and oxygen atom, satisfying the octet for oxygen and duet for hydrogen).
- Carbon Dioxide (CO₂): O=C=O (Double bonds represent the sharing of four electrons between carbon and each oxygen atom).
3. Coordinate Covalent Bonds (Dative Bonds): One Atom Donates Both Electrons
A coordinate covalent bond, or dative bond, is a special type of covalent bond where both electrons in the shared pair come from the same atom. This is often seen in molecules containing lone pairs of electrons. In Lewis structures, coordinate covalent bonds are sometimes represented by an arrow pointing from the electron-donating atom to the electron-accepting atom. For example, in the ammonium ion (NH₄⁺):
The nitrogen atom donates a lone pair of electrons to form a coordinate covalent bond with a hydrogen ion (H⁺).
Advanced Applications and Exceptions to the Octet Rule
While the octet rule provides a useful guideline, there are exceptions. Understanding these exceptions requires a deeper understanding of electron configurations and bonding principles.
1. Incomplete Octet:
Some elements, particularly those in the second period (like beryllium and boron), can have fewer than eight valence electrons in their stable compounds. This is often due to the small size and limited number of orbitals available.
2. Expanded Octet:
Elements in the third period and beyond can have more than eight valence electrons because they have access to d-orbitals that can accommodate additional electrons. This is common with elements like phosphorus and sulfur. For example, phosphorus pentachloride (PCl₅) has phosphorus surrounded by five chlorine atoms, resulting in ten valence electrons around the phosphorus.
3. Odd Electron Molecules (Free Radicals):
Molecules with an odd number of valence electrons cannot satisfy the octet rule for all atoms. These are called free radicals and are often highly reactive. Nitric oxide (NO) is a classic example.
4. Resonance Structures:
For some molecules, a single Lewis structure cannot accurately represent the bonding. In these cases, multiple Lewis structures, called resonance structures, are used to describe the delocalized electrons within the molecule. Benzene (C₆H₆) is a prime example where resonance structures are used to describe the delocalized pi electrons.
Formal Charge and its Significance in Lewis Structures
The concept of formal charge helps determine the most plausible Lewis structure when multiple possibilities exist. The formal charge of an atom is the difference between the number of valence electrons in the neutral atom and the number of electrons assigned to it in the Lewis structure.
Formal Charge = (Valence electrons) - (Non-bonding electrons) - ½(Bonding electrons)
A Lewis structure with the lowest formal charges on individual atoms is generally preferred. A formal charge of zero is ideal.
Importance of Lewis Formulas in Chemistry
Lewis dot structures are fundamental tools in chemistry, used for:
- Predicting molecular geometry: The arrangement of atoms and electron pairs influences a molecule's three-dimensional shape. Lewis structures provide the starting point for determining molecular geometry using VSEPR (Valence Shell Electron Pair Repulsion) theory.
- Understanding chemical reactivity: The presence of lone pairs, multiple bonds, or incomplete octets influences a molecule's reactivity.
- Determining polarity: The distribution of electrons in a molecule affects its polarity, which has implications for its physical and chemical properties.
- Illustrating reaction mechanisms: Lewis structures can be used to visualize the breaking and formation of bonds during chemical reactions.
- Teaching and learning: They provide a simple yet powerful visual representation of chemical bonding that aids in understanding complex chemical concepts.
Conclusion
The dots in a Lewis formula represent the valence electrons of an atom, which are the electrons involved in chemical bonding. These diagrams offer a simplified, yet powerful, visual representation of chemical bonding, enabling chemists to understand and predict the properties of molecules. While the octet rule serves as a useful guideline, it’s vital to understand the exceptions and advanced concepts like resonance structures and formal charge to accurately depict the bonding in a diverse range of molecules. Mastery of Lewis structures is crucial for any student or professional working within the realm of chemistry. They form the bedrock upon which much of our understanding of chemical behavior is built.
Latest Posts
Latest Posts
-
Difference Between Tlc And Column Chromatography
Mar 15, 2025
-
Energy Required To Remove An Electron From A Gaseous Atom
Mar 15, 2025
-
Que Es La Descomposicion De Acidos
Mar 15, 2025
-
Which Factor Affects Congressional Approval Ratings The Most
Mar 15, 2025
-
Fourier Transform Of A Differential Equation
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about In A Lewis Formula The Dots Represent . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
