In Coulomb's Law What Is K
Muz Play
Mar 20, 2025 · 5 min read
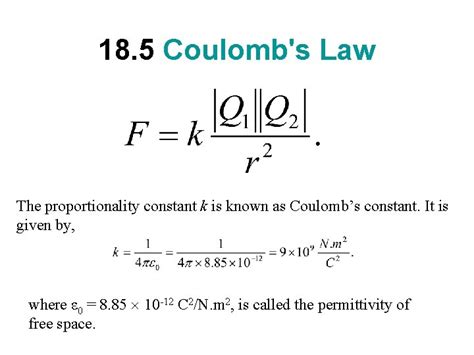
Table of Contents
In Coulomb's Law, What is k? A Deep Dive into Coulomb's Constant
Coulomb's Law is a fundamental principle in physics that describes the electrostatic interaction between electrically charged particles. It states that the force between two point charges is directly proportional to the product of their charges and inversely proportional to the square of the distance between them. Central to this law is a constant, k, often referred to as Coulomb's constant, the electrostatic constant, or the electric force constant. Understanding this constant is crucial to grasping the nuances of Coulomb's Law and its applications. This article will delve deep into the nature of k, exploring its value, units, derivation, and significance in various contexts.
What is Coulomb's Constant (k)?
Coulomb's constant (k) is a proportionality constant that appears in Coulomb's Law. It quantifies the strength of the electrostatic force between two charged particles. The law itself can be expressed mathematically as:
F = k * |q1 * q2| / r²
Where:
- F represents the electrostatic force between the two charges.
- k is Coulomb's constant.
- q1 and q2 are the magnitudes of the two charges.
- r is the distance separating the centers of the two charges.
The absolute value signs around q1 and q2 ensure that the force is always positive, representing the magnitude of the force. The direction of the force is determined by the signs of the charges: like charges repel (force is positive), and unlike charges attract (force is negative).
The Value of k and its Units
The value of k depends on the system of units used. In the International System of Units (SI), the most commonly used system, the value of k is approximately:
k ≈ 8.98755 × 10⁹ N⋅m²/C²
Where:
- N represents Newtons (the SI unit of force).
- m represents meters (the SI unit of distance).
- C represents Coulombs (the SI unit of electric charge).
This value reflects the strength of the electrostatic interaction in the SI system. The units of k (N⋅m²/C²) are chosen to ensure that the force F is expressed in Newtons when the charges are in Coulombs and the distance is in meters. The large magnitude of k highlights the considerable strength of the electrostatic force, particularly at short distances.
Derivation of Coulomb's Constant
The value of k isn't arbitrarily chosen; it's derived from other fundamental physical constants. It's related to the permittivity of free space (ε₀), a measure of how easily an electric field can be established in a vacuum. The relationship is:
k = 1 / (4πε₀)
The permittivity of free space (ε₀) has a precisely defined value in the SI system:
ε₀ ≈ 8.8541878128 × 10⁻¹² C²/N⋅m²
Substituting the value of ε₀ into the equation above gives the value of k we've already discussed. This connection shows that Coulomb's constant isn't an independent constant but is intrinsically linked to the fundamental properties of the vacuum.
The Significance of Coulomb's Constant
Coulomb's constant plays a vital role in various aspects of physics and related fields:
1. Understanding Electrostatic Interactions:
The most direct application of k is in calculating the force between charged particles. This is crucial in understanding atomic structure, molecular bonding, and the behavior of materials at a microscopic level. For instance, the constant helps explain why electrons orbit the nucleus of an atom and how molecules are formed through electrostatic attraction.
2. Electromagnetism and Maxwell's Equations:
Coulomb's Law is a cornerstone of classical electromagnetism and is incorporated into Maxwell's equations, a set of four equations that describe the behavior of electric and magnetic fields. k appears explicitly in Gauss's law for electricity, one of Maxwell's equations, which relates the electric flux through a closed surface to the enclosed electric charge.
3. Capacitance and Electric Potential:
Coulomb's constant is also critical in calculating capacitance, a measure of a capacitor's ability to store electrical energy. It features prominently in formulas for the capacitance of various capacitor geometries. Similarly, k is present in equations for calculating electric potential, the potential energy per unit charge at a given point in an electric field.
4. Chemical Bonding and Molecular Interactions:
In chemistry, Coulomb's Law, including its constant, is fundamental to understanding chemical bonding. The attraction between oppositely charged ions in ionic bonds and the interactions between molecules are governed by electrostatic forces, which are calculated using Coulomb's Law with the constant k. The strength of these interactions, and hence the properties of the resulting compounds, are directly influenced by the value of k.
5. Materials Science and Nanotechnology:
Coulomb's Law and its constant have significant implications in materials science and nanotechnology. The interactions between atoms and molecules in materials determine their physical and chemical properties. Understanding these interactions, which are largely governed by electrostatic forces, is critical for designing new materials with specific properties. This is particularly important at the nanoscale, where electrostatic forces play a more dominant role.
6. Particle Physics and Nuclear Physics:
Although Coulomb's Law is a classical theory, it provides a good approximation of the electrostatic interaction between charged particles even at the subatomic level. It plays a role in understanding the behavior of particles in particle accelerators and in nuclear physics, where the electrostatic repulsion between protons in the nucleus influences nuclear stability.
Variations and Considerations:
While the value of k provided earlier is for free space, it's important to note that the value changes when considering the presence of a dielectric material. Dielectric materials reduce the strength of the electrostatic force. The modified Coulomb's Law for a dielectric medium involves the relative permittivity (dielectric constant) of the material, effectively reducing the value of k.
Conclusion:
Coulomb's constant, k, is far more than just a number in an equation. It represents the strength of the fundamental electrostatic force governing the interactions between charged particles, influencing countless phenomena from the microscopic world of atoms and molecules to the macroscopic realm of materials and technologies. Its derivation from the permittivity of free space highlights its deep connection to the fundamental properties of the universe. Understanding the nature and significance of k is essential for a comprehensive grasp of electromagnetism and its profound impact across diverse scientific and technological disciplines. Further exploration into its implications in various fields continues to advance our understanding of the physical world around us.
Latest Posts
Latest Posts
-
Two Isotopes Of An Element Differ Only In Their
Mar 20, 2025
-
Chemical Potential And Gibbs Free Energy
Mar 20, 2025
-
Law Of Independent Assortment Vs Segregation
Mar 20, 2025
-
Effect Of Temperature On Bacterial Growth
Mar 20, 2025
-
Which Bond Is The Backbone Of All Protein Molecules
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about In Coulomb's Law What Is K . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
