Which Bond Is The Backbone Of All Protein Molecules
Muz Play
Mar 20, 2025 · 6 min read
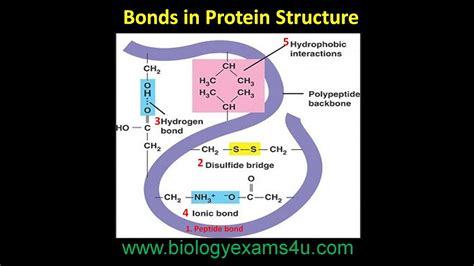
Table of Contents
Which Bond is the Backbone of All Protein Molecules?
The backbone of all protein molecules is formed by peptide bonds. This seemingly simple bond plays a crucial role in determining the three-dimensional structure and, consequently, the function of proteins, the workhorses of life. Understanding the peptide bond's properties is fundamental to comprehending the complexities of protein biochemistry and its vast implications in various fields, from medicine and biotechnology to materials science.
What is a Peptide Bond?
A peptide bond is a covalent bond formed between two amino acids. Specifically, it's an amide bond that joins the carboxyl group (-COOH) of one amino acid to the amino group (-NH2) of another amino acid. This reaction, called dehydration synthesis or condensation reaction, releases a water molecule (H₂O) as a byproduct. The resulting bond is a strong chemical link that holds the amino acid residues together.
The Chemistry of Peptide Bond Formation
The formation of a peptide bond involves several steps:
-
Activation of the carboxyl group: The carboxyl group of the first amino acid must be activated to make it more reactive. This often involves the attachment of a high-energy molecule like ATP (adenosine triphosphate).
-
Nucleophilic attack: The activated carboxyl group is then attacked by the lone pair of electrons on the nitrogen atom of the amino group of the second amino acid.
-
Bond formation: A new covalent bond is formed between the carbon atom of the carboxyl group and the nitrogen atom of the amino group.
-
Water molecule release: A water molecule is released during the bond formation.
The peptide bond is represented as -CO-NH-, and this repeating unit forms the protein backbone. The side chains (R groups) of the amino acids extend outward from the backbone, contributing significantly to the protein's overall structure and function.
The Importance of the Peptide Bond in Protein Structure
The peptide bond's characteristics are essential to protein structure and function:
-
Planar Geometry: Due to resonance between the carbonyl oxygen and the amide nitrogen, the peptide bond exhibits partial double-bond character. This restricts rotation around the peptide bond, forcing the six atoms involved (Cα-C-N-Cα) into a planar configuration. This planarity is a critical determinant of protein folding.
-
Partial Double Bond Character: The resonance contributes to the rigidity and stability of the peptide bond. It also impacts the bond length, which is shorter than a typical single bond, further influencing protein conformation.
-
Polarity: The peptide bond has a polar nature because of the electronegativity difference between the carbonyl oxygen and the amide nitrogen. This polarity contributes to hydrogen bonding, a crucial interaction in protein secondary structure formation (alpha-helices and beta-sheets).
-
Trans Configuration: In most peptide bonds, the two α-carbon atoms are positioned on opposite sides of the peptide bond (trans configuration). This configuration is energetically more favorable than the cis configuration where they are on the same side. However, certain proline residues can exist in the cis configuration, influencing local protein folding.
Peptide Bond and Protein Levels of Structure
The peptide bond is the foundation upon which all levels of protein structure are built:
1. Primary Structure: The Amino Acid Sequence
The primary structure of a protein refers to the linear sequence of amino acids linked by peptide bonds. This sequence, dictated by the genetic code, is crucial because it determines all higher-order structures. Even a single amino acid substitution can drastically alter a protein's function, as seen in sickle cell anemia.
2. Secondary Structure: Alpha-Helices and Beta-Sheets
Secondary structures are local spatial arrangements of the polypeptide chain stabilized by hydrogen bonds between the backbone amide and carbonyl groups. Alpha-helices and beta-sheets are the most common secondary structures. The planarity of the peptide bond and its capacity to form hydrogen bonds are key to the stability of these structures.
-
Alpha-Helices: A right-handed coiled structure stabilized by hydrogen bonds between the carbonyl oxygen of one amino acid and the amide hydrogen of an amino acid four residues down the chain.
-
Beta-Sheets: Formed by hydrogen bonding between adjacent polypeptide chains (or segments of the same chain folded back on itself). These can be parallel or anti-parallel, depending on the orientation of the polypeptide chains.
3. Tertiary Structure: 3D Folding
The tertiary structure describes the overall three-dimensional arrangement of a polypeptide chain, including the spatial relationships between its secondary structure elements. This folding is driven by various interactions, including:
-
Hydrophobic interactions: Nonpolar amino acid side chains cluster together in the protein's core, away from the aqueous environment.
-
Hydrogen bonds: These bonds form between various parts of the polypeptide chain, stabilizing the folded structure.
-
Ionic interactions: Electrostatic attractions between charged amino acid side chains.
-
Disulfide bonds: Covalent bonds formed between cysteine residues, providing additional stability.
The peptide bond's rigidity and its ability to participate in hydrogen bonding are fundamental to the intricate folding pattern of the tertiary structure. This folding is crucial for protein function, as it creates specific binding sites for ligands, substrates, or other proteins.
4. Quaternary Structure: Multiple Polypeptide Chains
Some proteins consist of multiple polypeptide chains (subunits) assembled into a functional complex. This arrangement is called the quaternary structure. The interactions between subunits are similar to those stabilizing tertiary structure. The peptide bond remains the fundamental unit holding each individual polypeptide chain together.
Peptide Bond Cleavage: Enzymatic Hydrolysis
The peptide bond, while strong, can be cleaved through enzymatic hydrolysis. This process is crucial in various biological processes, including protein digestion and protein degradation. Proteases, a class of enzymes, catalyze the hydrolysis of peptide bonds, breaking down proteins into smaller peptides or individual amino acids. The specificity of different proteases allows for precise control over protein degradation within the cell.
Applications and Significance of Understanding Peptide Bonds
Understanding the peptide bond's properties and its role in protein structure has profound implications in numerous fields:
-
Drug Design: Many drugs target specific proteins. Knowledge of peptide bonds and protein folding allows scientists to design drugs that inhibit or activate protein function by interfering with peptide bond interactions or protein folding.
-
Biotechnology: Recombinant protein production relies on understanding peptide bonds. Genetic engineering techniques are used to produce proteins with specific sequences, and proper folding of the protein is crucial for its function.
-
Materials Science: Peptide-based materials are being developed for various applications, including biomaterials, drug delivery systems, and tissue engineering. The strength and biocompatibility of peptide bonds make them attractive for these applications.
-
Proteomics: The study of proteomes (the entire set of proteins expressed by an organism) relies heavily on techniques that identify and analyze proteins, including the identification of peptide fragments.
-
Medical Diagnostics: Analyzing peptide fragments resulting from protein degradation can provide valuable information about disease states. For instance, specific peptide biomarkers can indicate the presence of certain cancers or other diseases.
Conclusion
The peptide bond is the incontestable backbone of all protein molecules. Its unique chemical properties—planarity, partial double-bond character, and polarity—are essential determinants of protein structure and function. From the primary amino acid sequence to the complex three-dimensional folding of proteins, the peptide bond plays a fundamental role. Understanding this seemingly simple bond is key to comprehending the complexities of life and its vast implications in various scientific and technological fields. Continued research into peptide bonds and their interactions will undoubtedly lead to further advancements in medicine, biotechnology, and other disciplines. Further exploration into peptide bond modifications, such as isomerization and cyclization, also promises exciting possibilities in the design and application of novel peptide-based therapeutics and materials.
Latest Posts
Latest Posts
-
Speak Out Call In Public Speaking As Advocacy
Mar 21, 2025
-
How To Tell If A Reaction Is Redox
Mar 21, 2025
-
Most Blood Enters The Ventricle During
Mar 21, 2025
-
Whats The Atomic Number For Helium
Mar 21, 2025
-
Is Burning A Chemical Or Physical Change
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about Which Bond Is The Backbone Of All Protein Molecules . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
