What's The Atomic Number For Helium
Muz Play
Mar 21, 2025 · 6 min read
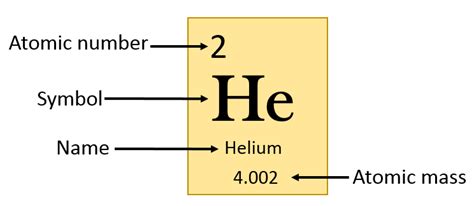
Table of Contents
What's the Atomic Number for Helium? A Deep Dive into the Noble Gas
Helium, the second-lightest and second-most abundant element in the universe, holds a unique position in the periodic table. Understanding its properties, especially its atomic number, is key to appreciating its significance in various scientific fields and everyday applications. So, what is the atomic number for helium? The answer, simply put, is 2. But let's delve much deeper than that simple answer, exploring the meaning of atomic number, helium's unique characteristics stemming from this number, and its wide-ranging implications.
Understanding Atomic Number
The atomic number of an element represents the number of protons found in the nucleus of a single atom of that element. This number is fundamental because it uniquely identifies each element. No two elements share the same atomic number. While the number of neutrons (in isotopes) and electrons (in ions) can vary, the number of protons remains constant, defining the element's identity.
The Significance of Protons
Protons, positively charged particles within the atomic nucleus, determine an atom's chemical behavior. The number of protons dictates the number of electrons an atom will have in its neutral state, which, in turn, defines the atom's electron configuration and its reactivity. Helium, with its atomic number of 2, possesses two protons in its nucleus. This seemingly small number has profound implications for its properties.
Helium's Unique Properties: A Consequence of Atomic Number 2
Helium's atomic number directly contributes to its distinctive characteristics, which set it apart from other elements.
Inertness and Chemical Stability: The Noble Gas Family
Helium, along with neon, argon, krypton, xenon, and radon, belongs to the group of noble gases. These elements are characterized by their extremely low reactivity. This inertness is a direct consequence of their electron configuration. Helium, with its two electrons filling its first electron shell completely, achieves a stable electron configuration known as a closed shell or duet. This stable configuration renders helium incredibly unreactive, making it chemically inert under normal conditions. This chemical stability is crucial for many of its applications.
Low Boiling Point and Density: Implications for Cryogenics
Helium possesses the lowest boiling point of all elements (-268.93 °C or 4.22 K). This exceptionally low boiling point, linked to its weak interatomic forces due to its electron configuration and small atomic size, makes it invaluable as a cryogenic refrigerant. Liquid helium is essential in applications like superconducting magnets in MRI machines and research involving extremely low temperatures. Its low density, also a consequence of its atomic structure, adds to its suitability for various applications.
Low Reactivity: Diverse Applications
Helium's low reactivity makes it ideal for applications where inertness is crucial. It's used as a shielding gas in welding to protect the weld from oxidation, as a pressurizing agent in rocket fuels, and in various analytical instruments to prevent unwanted chemical reactions. Its inert nature ensures that it doesn't interfere with the processes in which it's employed.
Unique Spectroscopic Properties: Identifying Helium
Helium's electron configuration also gives it unique spectroscopic properties. When excited, helium atoms emit light at specific wavelengths, producing a characteristic spectrum. This spectral signature allows scientists to identify and quantify helium in various samples, from astronomical observations to analyzing the composition of gases in industrial settings. The distinct spectral lines are directly tied to the energy level transitions of its two electrons.
The Discovery of Helium and its Atomic Number Determination
The discovery of helium is an interesting story that highlights the importance of spectroscopic analysis in identifying new elements. Helium was first detected in the Sun's spectrum during a solar eclipse in 1868, before its discovery on Earth. Its spectral lines, unique to helium, indicated the presence of an unknown element. Only later was it isolated and identified on Earth. The determination of its atomic number came with the development of understanding of atomic structure and the periodic table. The systematic organization of elements based on their properties, including their atomic number, allowed for the definitive placement of helium with its atomic number of 2.
Helium's Abundance and Distribution: A Cosmic Perspective
Helium's abundance in the universe is remarkable, second only to hydrogen. It's a product of nuclear fusion in stars, where hydrogen atoms fuse to form helium, releasing vast amounts of energy. This process is the primary energy source of stars, including our Sun. Helium's abundance in the universe underscores its importance in cosmic processes and stellar evolution. Understanding its atomic number is crucial in modeling these processes and understanding the composition of stars and interstellar matter.
Helium's Applications: A Broad Spectrum
Helium's unique properties translate into a wide range of applications across numerous sectors.
Scientific Research
- Cryogenics: Liquid helium is indispensable in various cryogenic applications, including MRI machines, superconducting magnets in particle accelerators, and low-temperature research.
- Spectroscopy: Helium's unique spectral lines are used in analytical techniques to identify and quantify helium in samples.
- Mass Spectrometry: Helium is used as a carrier gas in mass spectrometry to facilitate the analysis of molecules.
Industrial Applications
- Welding: Helium is used as a shielding gas in welding to protect the weld from atmospheric contamination.
- Leak Detection: Helium's small atomic size and low density make it an ideal gas for leak detection in various systems.
- Pressure Testing: Helium is used as a pressurizing agent in various industrial processes, including rocket fuel tanks.
Medical Applications
- MRI: Superconducting magnets in MRI machines rely on liquid helium to achieve the necessary low temperatures.
- Breathing Mixtures: Helium-oxygen mixtures are used in deep-sea diving and other applications where reduced breathing resistance is beneficial.
Other Applications
- Balloons: Helium is widely used to inflate balloons due to its low density and inertness.
- Arc Welding: It's used as a shielding gas in arc welding processes.
The Future of Helium: Conservation and Sustainability
Helium is a non-renewable resource, with limited sources on Earth. As its demand increases across various applications, the need for conservation and sustainable practices becomes paramount. Research into alternative technologies and methods for helium recovery and recycling is crucial for ensuring the long-term availability of this valuable element.
Conclusion: The Importance of Atomic Number 2
Helium's atomic number, 2, is the foundation upon which all its remarkable properties are built. From its chemical inertness to its low boiling point and unique spectroscopic characteristics, everything about helium stems from this fundamental number. Understanding its atomic number is key to appreciating its significance in various scientific, industrial, and medical applications, and to ensuring its sustainable use for future generations. Its role extends beyond Earth, playing a critical role in stellar processes and the overall composition of the universe. The simple number 2 holds a profound significance in understanding both the microcosm of atomic structure and the macrocosm of the cosmos.
Latest Posts
Latest Posts
-
The Si Unit Of Energy Is The
Mar 28, 2025
-
Water Molecules Move Across Cells By
Mar 28, 2025
-
Is Ammonium Hydroxide A Strong Base
Mar 28, 2025
-
What Is The Activity Series In Chemistry
Mar 28, 2025
-
Solubility Of A Gas In A Liquid
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about What's The Atomic Number For Helium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
