Solubility Of A Gas In A Liquid
Muz Play
Mar 28, 2025 · 6 min read
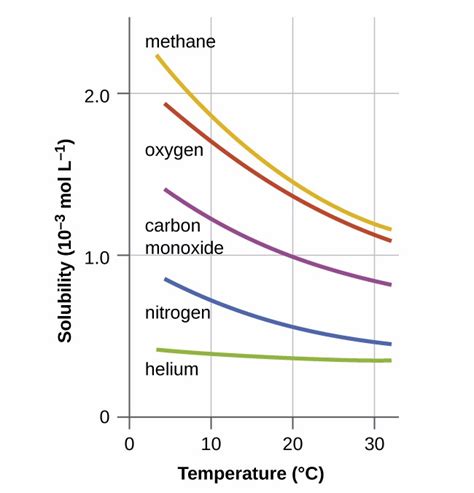
Table of Contents
The Solubility of Gases in Liquids: A Deep Dive
The solubility of a gas in a liquid is a fundamental concept in chemistry with widespread applications in various fields, from environmental science and biochemistry to chemical engineering and medicine. Understanding the factors that influence gas solubility is crucial for predicting and controlling processes involving gas-liquid interactions. This article provides a comprehensive overview of gas solubility in liquids, exploring the underlying principles, influencing factors, and practical implications.
What is Gas Solubility?
Gas solubility refers to the maximum amount of gas that can dissolve in a given amount of liquid at a specific temperature and pressure. It's a measure of how much gas can be incorporated into the liquid's structure before reaching saturation. Unlike solids and liquids, which have relatively fixed structures, gases readily dissolve and escape from solution depending on the prevailing conditions. This dynamic equilibrium between dissolved gas and gas in the gaseous phase is central to understanding gas solubility.
Understanding the Dissolution Process
The dissolution of a gas in a liquid is a physical process, not a chemical reaction. Gas molecules, initially free-moving in the gaseous phase, collide with the liquid's surface. If the kinetic energy of the gas molecule is low enough and there's an available space in the liquid's structure, the gas molecule can be trapped within the liquid phase. This is governed by intermolecular forces between the gas molecules and the liquid molecules. Stronger interactions lead to higher solubility.
Factors Affecting Gas Solubility
Several factors significantly impact the solubility of a gas in a liquid. These factors are intricately linked and need to be considered collectively to gain a complete picture.
1. Partial Pressure of the Gas (Henry's Law)
Henry's Law is a cornerstone of gas solubility. It states that the amount of gas dissolved in a liquid is directly proportional to the partial pressure of that gas above the liquid. Mathematically, it's represented as:
C = kH * P
where:
- C is the concentration of the dissolved gas
- kH is Henry's Law constant (specific to the gas-liquid pair and temperature)
- P is the partial pressure of the gas above the liquid.
This law implies that increasing the partial pressure of a gas above a liquid will increase the amount of gas dissolved. This principle is utilized in carbonated beverages where CO2 is dissolved under high pressure. When the bottle is opened, the pressure decreases, leading to the release of CO2 as bubbles.
2. Temperature
Temperature plays a crucial role in gas solubility. Generally, the solubility of gases in liquids decreases with increasing temperature. This is because higher temperatures provide gas molecules with more kinetic energy, allowing them to overcome the intermolecular forces holding them in solution and escape into the gaseous phase. Think of boiling water – the dissolved air escapes as the temperature increases. This inverse relationship is not absolute, but it holds true for most gas-liquid systems.
3. Nature of the Solvent and Solute
The chemical nature of both the gas and the liquid significantly impacts solubility. Polar solvents tend to dissolve polar gases more readily, while nonpolar solvents dissolve nonpolar gases better. This is due to the principle of "like dissolves like." For example, oxygen (a nonpolar gas) is more soluble in nonpolar solvents like oil compared to polar solvents like water.
The size and shape of the gas molecules also affect solubility. Smaller gas molecules generally have higher solubility than larger ones because they can more easily fit into the spaces within the liquid structure.
4. Presence of Other Dissolved Substances
The presence of other dissolved substances can influence gas solubility. Salts and other electrolytes often decrease the solubility of gases in water, a phenomenon known as the salting-out effect. This is because the dissolved ions interact with the water molecules, reducing their ability to interact with and dissolve gas molecules. Conversely, some substances can increase gas solubility.
5. Intermolecular Forces
The strength of intermolecular forces between gas molecules and liquid molecules is paramount. Stronger attractive forces between gas and liquid molecules lead to increased solubility. These forces can include dipole-dipole interactions, hydrogen bonding, and London dispersion forces. The presence of strong hydrogen bonding in water, for example, allows it to dissolve some gases to a certain extent, despite being a polar molecule.
Applications of Gas Solubility
Understanding and controlling gas solubility is essential in numerous applications across various disciplines:
1. Environmental Science
Gas solubility is critical in understanding atmospheric processes, such as the dissolution of CO2 in oceans, affecting marine life and ocean acidification. It also plays a crucial role in assessing water quality and pollution levels, particularly concerning the solubility of harmful gases like sulfur dioxide and nitrogen oxides.
2. Chemical Engineering
Gas solubility is crucial in designing and optimizing various industrial processes. Examples include:
- Absorption: Removing unwanted gases from gas streams using liquid solvents.
- Stripping: Removing dissolved gases from liquids.
- Aeration: Introducing gases into liquids, such as oxygen in wastewater treatment.
3. Biochemistry and Medicine
Gas solubility is fundamental to biological processes. The transport of oxygen in blood, for example, relies heavily on the solubility of oxygen in blood plasma and its binding to hemoglobin. In medicine, controlled gas delivery systems leverage solubility principles to administer therapeutic gases. Anesthesia, for instance, relies on the solubility of anesthetic gases in the bloodstream and tissues.
4. Food and Beverage Industry
The solubility of gases is key to creating carbonated drinks, where CO2 is dissolved under pressure, and in brewing, where the solubility of CO2 influences the carbonation of beer. The packaging and storage of food products also require consideration of gas solubility to prevent spoilage caused by oxygen or other gases.
5. Material Science
Gas solubility plays a vital role in material science in the creation of gas-filled polymers and other materials with controlled gas permeability. This is particularly relevant in packaging, drug delivery systems, and the development of sensors.
Advanced Concepts and Considerations
Beyond the basic principles, several more sophisticated concepts relate to gas solubility:
- Activity Coefficients: In concentrated solutions, the concept of activity coefficients corrects for deviations from ideal behavior, providing a more accurate representation of gas solubility.
- Non-ideal Solutions: Henry's Law is based on ideal solutions. Real solutions deviate from ideal behavior, especially at higher concentrations, necessitating more complex models for accurate prediction.
- Temperature Dependence of Henry's Law Constant: The Henry's Law constant (kH) is strongly temperature-dependent. Empirical equations or thermodynamic models are often used to describe this dependence.
- Solubility of Gas Mixtures: When dealing with mixtures of gases, the solubility of each gas is determined by its individual partial pressure, independent of the presence of other gases (assuming ideal behavior).
Conclusion
Gas solubility is a multifaceted concept governed by several interacting factors. Understanding these factors and the underlying principles is critical for various applications across diverse fields. From environmental science and chemical engineering to biochemistry and medicine, the principles of gas solubility are indispensable tools for researchers, engineers, and scientists alike. The accurate prediction and control of gas solubility remain a focus of ongoing research, with advancements continually refining our understanding and enabling innovative applications. Future research will likely focus on developing more accurate models for non-ideal systems and exploring the implications of gas solubility in emerging technologies.
Latest Posts
Latest Posts
-
What Happens To An Animal Cell In A Isotonic Solution
Mar 31, 2025
-
Atom That Has Gained Or Lost Electrons
Mar 31, 2025
-
Cuantas Onzas Tiene Un Cuarto De Galon
Mar 31, 2025
-
Work Done By A Varying Force
Mar 31, 2025
-
The Rna Components Of Ribosomes Are Synthesized In The
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Solubility Of A Gas In A Liquid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
