In Mass Spectrometry What Is The Base Peak
Muz Play
Mar 23, 2025 · 6 min read
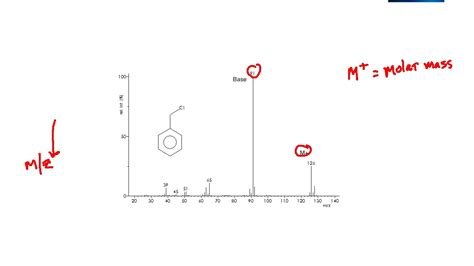
Table of Contents
In Mass Spectrometry: What is the Base Peak? A Comprehensive Guide
Mass spectrometry (MS) is a powerful analytical technique used to identify and quantify the components within a sample. It works by ionizing molecules, separating them based on their mass-to-charge ratio (m/z), and then detecting them. Within a mass spectrum, a wealth of information is encoded, and understanding key features like the base peak is crucial for accurate interpretation. This article will delve deep into the concept of the base peak in mass spectrometry, exploring its definition, significance, and applications.
Understanding the Mass Spectrum
Before diving into the base peak, let's briefly recap what a mass spectrum is. A mass spectrum is a graphical representation of the relative abundance of ions as a function of their mass-to-charge ratio (m/z). The x-axis represents the m/z value, indicating the mass of the ion relative to its charge. The y-axis represents the relative abundance or intensity of each ion, usually expressed as a percentage or arbitrary units. The spectrum shows a series of peaks, each corresponding to a specific ion with a unique m/z value.
Defining the Base Peak
The base peak is simply the most abundant ion in a mass spectrum. It's the peak with the highest intensity, representing the ion that is most readily formed during the ionization process and survives the journey through the mass analyzer. This peak is assigned a relative abundance of 100%, and the intensities of all other peaks are reported relative to the base peak. This standardization facilitates comparison between different spectra, even if the total ion counts vary.
Why is the Base Peak Important?
The base peak holds significant value for several reasons:
-
Identification of Compounds: The m/z value of the base peak often corresponds to a significant fragment or the molecular ion (if present) of the analyte. This can serve as a crucial clue in identifying the compound. While not always the molecular ion, its presence often aids in structural elucidation.
-
Structural Elucidation: The base peak, along with other prominent peaks, helps in deducing the structure of the molecule. The fragmentation patterns observed in the mass spectrum, including the formation of the base peak, provide valuable information about the functional groups and bonding present in the analyte.
-
Quantitative Analysis: Though not the primary focus, the intensity of the base peak relative to other peaks can aid in quantitative analysis, especially when using methods such as selected ion monitoring (SIM).
-
Comparison of Spectra: Because the intensities of all peaks are normalized relative to the base peak, it simplifies the comparison of spectra from different samples or experiments. Even if the overall sensitivity changes, the relative abundance of different ions remains consistent, allowing for easier comparison.
-
Instrument Performance Monitoring: The intensity of the base peak can be used as an indicator of instrument performance and stability. Significant changes in the base peak intensity could suggest issues with the ionization source or detector.
Factors Influencing the Base Peak
Several factors influence which ion becomes the base peak in a mass spectrum:
-
Ionization Method: Different ionization techniques (e.g., Electron Ionization (EI), Chemical Ionization (CI), Electrospray Ionization (ESI), Matrix-Assisted Laser Desorption/Ionization (MALDI)) produce distinct fragmentation patterns, leading to different base peaks. EI, for example, often produces abundant fragment ions, while ESI tends to produce more intact molecular ions.
-
Molecular Structure: The chemical structure of the analyte plays a crucial role. Certain functional groups are more prone to fragmentation than others, influencing which fragments are most abundant. The presence of weak bonds can lead to the formation of characteristic base peaks.
-
Instrument Parameters: Instrument parameters like the source temperature, voltage settings, and collision energy can affect the fragmentation process and, consequently, the base peak. Optimizing these parameters can enhance the signal of specific fragments, including the base peak.
-
Sample Purity: Impurities in the sample can lead to additional peaks and interfere with the identification of the base peak from the target analyte. Proper sample preparation and purification are therefore essential.
Practical Applications of the Base Peak
The base peak finds utility across diverse applications:
-
Forensic Science: Mass spectrometry is extensively used in forensic science for identifying drugs, explosives, and other substances. The base peak can be a valuable marker in identifying and quantifying these compounds.
-
Environmental Monitoring: Mass spectrometry is employed for detecting pollutants and contaminants in environmental samples like water, air, and soil. The base peak helps in identifying and quantifying these pollutants.
-
Pharmaceutical Analysis: In pharmaceutical research and development, mass spectrometry aids in characterizing drugs, metabolites, and impurities. The base peak assists in identifying and quantifying these compounds.
-
Clinical Diagnostics: Mass spectrometry is utilized in clinical diagnostics for analyzing biological samples like blood and urine to detect diseases. The base peak can be a valuable marker for different diseases or conditions.
-
Food Safety: Mass spectrometry is used to detect contaminants and adulterants in food products. The base peak helps in identifying the contaminants and ensuring food safety.
-
Proteomics and Metabolomics: Mass spectrometry is a cornerstone of proteomics (the study of proteins) and metabolomics (the study of metabolites). The base peak helps in the identification and quantification of proteins and metabolites, crucial for understanding biological processes.
Distinguishing the Base Peak from the Molecular Ion Peak
It's important to differentiate the base peak from the molecular ion peak (M+•). While both are significant features of a mass spectrum, they are not always the same.
The molecular ion peak represents the ion formed by the removal of a single electron from the neutral molecule. Its m/z value corresponds to the molecular weight of the compound. However, the molecular ion's intensity is not always the highest; it can be weak or even absent depending on the molecule's structure and the ionization method used.
The base peak, as stated, is the most abundant ion regardless of its structure or origin. It can be a fragment ion, an adduct ion, or even, sometimes, the molecular ion.
Example: Imagine a mass spectrum of a compound. The molecular ion peak (M+•) might have an m/z value of 150, but its intensity might be only 20%. A fragment ion with an m/z value of 100 might have an intensity of 100%, making it the base peak.
Advanced Techniques and the Base Peak
In advanced mass spectrometry techniques like tandem mass spectrometry (MS/MS), the base peak in the product ion spectrum provides additional information about the structure of the precursor ion. The fragmentation of a selected precursor ion in MS/MS generates a product ion spectrum, and the base peak in this spectrum represents the most abundant fragment ion generated from that precursor. This detailed information enhances structural elucidation significantly.
Conclusion
The base peak is a critical element in mass spectrometry, offering valuable insight into the composition and structure of an analyte. Its identification, combined with other spectral features, plays a pivotal role in compound identification, structural elucidation, quantitative analysis, and a range of applications across various scientific disciplines. Understanding the factors influencing the base peak and its relationship to the molecular ion peak is crucial for the accurate interpretation of mass spectra and the effective application of mass spectrometry in diverse fields. The continued development of mass spectrometry techniques and data analysis tools will further enhance the importance and utility of the base peak in future applications.
Latest Posts
Latest Posts
-
Rearrangement Of Benzil To Benzilic Acid
Mar 25, 2025
-
Is Table Salt A Mixture Or Pure Substance
Mar 25, 2025
-
How To Do Inverse Laplace Transforms
Mar 25, 2025
-
Real World Application Of A Linear Equation In 2 Variables
Mar 25, 2025
-
Is Boil A Physical Or Chemical Change
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about In Mass Spectrometry What Is The Base Peak . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
