Is A Nonmetal A Noble Gas
Muz Play
Mar 26, 2025 · 5 min read
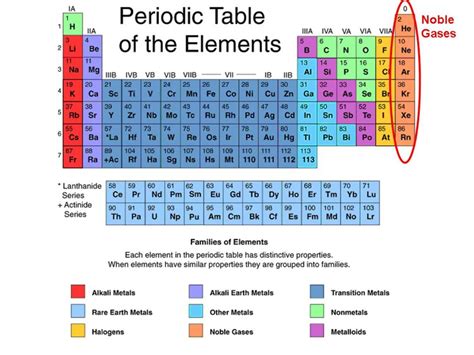
Table of Contents
Is a Nonmetal a Noble Gas? Understanding the Differences and Similarities
The periodic table organizes elements based on their properties, and two key categories are nonmetals and noble gases. While both groups share some similarities, particularly in their gaseous states under standard conditions, they are fundamentally different in their chemical behavior and electron configurations. This article delves deep into the distinctions and subtle overlaps between nonmetals and noble gases, clarifying whether a nonmetal can be classified as a noble gas.
Defining Nonmetals
Nonmetals are a diverse group of elements located on the right side of the periodic table. Their defining characteristic is their lack of metallic properties. They typically exhibit:
- Low electrical conductivity: They are poor conductors of electricity.
- Low thermal conductivity: They don't readily transfer heat.
- High electronegativity: They tend to attract electrons in chemical bonds.
- Brittle solid state (when solid): They lack the malleability and ductility of metals.
- Varied physical states: Nonmetals can exist as solids (like carbon), liquids (like bromine), or gases (like oxygen) at room temperature.
Examples of common nonmetals include:
- Hydrogen (H): Unique in its position on the periodic table, often considered a nonmetal due to its properties.
- Carbon (C): Forms the basis of organic chemistry and exists in various allotropes (diamond, graphite).
- Nitrogen (N): A crucial component of the Earth's atmosphere and a building block for amino acids.
- Oxygen (O): Essential for respiration and forms a large portion of the Earth's atmosphere.
- Phosphorus (P): Used in fertilizers and matches.
- Sulfur (S): Used in vulcanization of rubber and the production of sulfuric acid.
- Selenium (Se): Used in photocopiers and solar cells.
- Chlorine (Cl): Used as a disinfectant and in the production of PVC.
- Bromine (Br): A reddish-brown liquid element.
- Iodine (I): Used in antiseptic solutions.
- Astatine (At): A radioactive and rare element.
Understanding Noble Gases
Noble gases, also known as inert gases, are located in Group 18 of the periodic table. Their defining characteristic is their extreme chemical inertness. This is due to their:
- Complete valence electron shells: They possess a full outer electron shell, giving them exceptional stability. This full outer shell renders them highly unreactive. They have little tendency to gain, lose, or share electrons to form chemical bonds.
- High ionization energies: It requires a significant amount of energy to remove an electron from a noble gas atom.
- Low electronegativity: They have a low tendency to attract electrons.
- Typically gaseous at room temperature: All noble gases are gases under standard conditions.
Examples of noble gases include:
- Helium (He): Used in balloons and cryogenics.
- Neon (Ne): Used in neon signs.
- Argon (Ar): Used in welding and as an inert atmosphere.
- Krypton (Kr): Used in some lighting applications.
- Xenon (Xe): Used in some lighting and medical applications.
- Radon (Rn): A radioactive gas.
- Oganesson (Og): A synthetic and highly radioactive element.
Key Differences: Nonmetals vs. Noble Gases
The most crucial distinction lies in their reactivity. While nonmetals are generally reactive, forming various compounds, noble gases are exceptionally unreactive. Their complete valence electron shells make them exceptionally stable, resisting chemical bonding.
| Feature | Nonmetals | Noble Gases |
|---|---|---|
| Reactivity | Generally reactive | Extremely unreactive |
| Valence Electrons | Incomplete outer electron shell | Complete outer electron shell (octet rule) |
| Electronegativity | Generally high | Low |
| Ionization Energy | Varies, generally moderate | High |
| Physical States | Solids, liquids, and gases at room temperature | Gases at room temperature |
| Chemical Bonding | Form covalent bonds, ionic bonds, etc. | Rarely form compounds (exception: xenon) |
Can a Nonmetal be a Noble Gas?
The simple answer is no. A nonmetal cannot simultaneously be a noble gas. These are distinct categories defined by fundamental differences in their electronic structure and chemical behavior. A nonmetal, by definition, has an incomplete valence electron shell, making it chemically reactive. A noble gas, on the other hand, has a complete valence shell, resulting in exceptional chemical inertness.
While some nonmetals may exist as gases under standard conditions (like oxygen and nitrogen), this is not a defining feature of noble gases. The defining characteristic is their extreme unreactivity stemming from their complete electron shells.
Exceptions and Nuances: The Case of Xenon
While noble gases are renowned for their unreactivity, xenon is a notable exception. Under specific, high-energy conditions, xenon can form compounds with highly electronegative elements like fluorine and oxygen. These compounds are exceptionally rare and unstable, highlighting the exceptional stability of the noble gas configuration. The formation of xenon compounds does not, however, reclassify xenon as a nonmetal. It remains a noble gas, simply exhibiting an unusual exception to its generally inert nature. This exception reinforces the fundamental difference between noble gases and nonmetals; while there might be rare exceptions to the rule of complete inertness among noble gases, the core characteristic remains their inherently stable electron configuration.
The Importance of Electron Configuration
The electronic configuration is paramount in understanding the differences between nonmetals and noble gases. Nonmetals strive to achieve a stable electron configuration, often by gaining, losing, or sharing electrons. Noble gases, already possessing a stable configuration, have no such drive to react. This fundamental difference in electron structure dictates their drastically different chemical properties. It’s the foundational principle that separates these two key groups within the periodic table's organization of elements.
Conclusion: Distinct Categories with Overlapping Characteristics
Although some nonmetals can exist as gases at standard temperatures, and although a very limited number of compounds have been made using xenon, the fundamental difference between nonmetals and noble gases remains. The crucial distinction lies in the completeness of their outer electron shells and the resulting differences in reactivity. Nonmetals, with their incomplete valence shells, actively participate in chemical reactions, while noble gases, possessing complete shells, exhibit remarkable inertness. Therefore, a nonmetal cannot be classified as a noble gas. The overlapping characteristics of gaseous state at room temperature or extremely rare compound formation should not be mistaken for a fundamental similarity in their chemical behavior and electronic structure. The periodic table's classification reflects these essential differences in a way that provides a clear and unambiguous organization of elements.
Latest Posts
Latest Posts
-
How To Find Point Of Tangency
Mar 29, 2025
-
How Do You Calculate Potential Difference
Mar 29, 2025
-
How To Round To Four Decimal Places
Mar 29, 2025
-
Reaction Of Benzoic Acid And Naoh
Mar 29, 2025
-
What Type Of Compounds Dissolve To Become Electrolyte
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Is A Nonmetal A Noble Gas . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
