Is A Proton Contribute Mass Of An Atom
Muz Play
Mar 15, 2025 · 6 min read
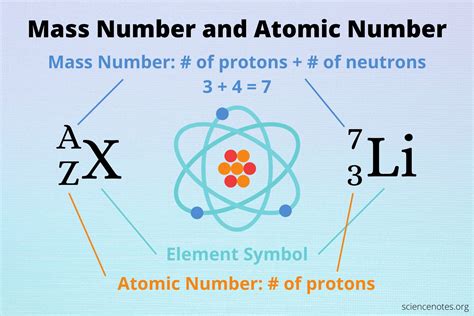
Table of Contents
Does a Proton Contribute to the Mass of an Atom? A Deep Dive into Atomic Structure and Mass
The simple answer is a resounding yes. Protons are a significant contributor to the overall mass of an atom. However, understanding how they contribute, and the nuances involved, requires a deeper delve into the fascinating world of atomic structure and the fundamental forces governing the universe. This article will explore the role of protons in atomic mass, contrasting them with neutrons and electrons, and touching upon the concepts of isotopes and atomic mass units.
Understanding Atomic Structure: The Three Main Subatomic Particles
Atoms, the fundamental building blocks of matter, are composed of three primary subatomic particles: protons, neutrons, and electrons. Each plays a crucial role in determining an atom's properties and behavior.
Protons: The Positively Charged Core
Protons reside in the atom's nucleus, the dense central region. They carry a positive electrical charge, equal in magnitude but opposite in sign to the electron's negative charge. Crucially, the number of protons in an atom's nucleus defines its atomic number, which uniquely identifies the element. For example, hydrogen (H) has one proton, helium (He) has two, and so on.
Key characteristics of protons:
- Positive charge: +1 elementary charge
- Mass: Approximately 1.6726 × 10^-27 kg (or 1 atomic mass unit, amu, approximately)
- Location: Nucleus
- Contribution to atomic number: Defines the element
Neutrons: The Neutral Nuclear Partners
Neutrons, like protons, are found within the atom's nucleus. As their name suggests, they carry no electrical charge (neutral). They play a vital role in nuclear stability, helping to bind protons together and preventing the electrostatic repulsion between positively charged protons from causing the nucleus to break apart.
Key characteristics of neutrons:
- No charge: Neutral
- Mass: Approximately 1.6749 × 10^-27 kg (slightly larger than a proton, also approximately 1 amu)
- Location: Nucleus
- Contribution to atomic mass: Significant contributor, influencing isotopes
Electrons: The Orbiting Negatively Charged Particles
Electrons are significantly lighter than protons and neutrons and reside in orbitals surrounding the nucleus. They carry a negative electrical charge, equal in magnitude to the proton's positive charge. The arrangement of electrons in these orbitals determines the chemical properties of an atom and its ability to form chemical bonds with other atoms.
Key characteristics of electrons:
- Negative charge: -1 elementary charge
- Mass: Approximately 9.1094 × 10^-31 kg (negligible compared to protons and neutrons)
- Location: Orbitals surrounding the nucleus
- Contribution to atomic mass: Negligible
How Protons Contribute to Atomic Mass: The Dominant Factor
The mass of an atom is primarily determined by the combined mass of its protons and neutrons. Electrons contribute so little to the overall mass that their contribution is often ignored in calculations. The total number of protons and neutrons is known as the mass number of the atom.
Let's consider an example: a carbon-12 atom (¹²C). This isotope has 6 protons and 6 neutrons. Therefore, its mass number is 12. The mass of the electrons is negligible in comparison to the protons and neutrons, meaning the mass of the ¹²C atom is approximately 12 amu.
The significance of protons' mass in the overall atomic mass:
- Direct proportionality: The more protons an atom has, the greater its mass.
- Dominant factor (along with neutrons): The mass of protons and neutrons accounts for virtually all of an atom's mass.
- Atomic mass unit (amu): The amu is defined based on the mass of a carbon-12 atom, highlighting the importance of protons and neutrons.
Isotopes: Variations in Neutron Number, but Constant Proton Number
Isotopes are atoms of the same element that have the same number of protons (and thus the same atomic number) but differ in the number of neutrons. This means they have the same chemical properties but different atomic masses. For example, carbon-12 (¹²C), carbon-13 (¹³C), and carbon-14 (¹⁴C) are all isotopes of carbon. They all have 6 protons, but they have 6, 7, and 8 neutrons, respectively.
While the number of protons remains constant for a given element, the variation in neutron number significantly impacts the atomic mass. This is because neutrons, despite being electrically neutral, possess a mass comparable to that of a proton. The differing mass numbers of isotopes stem directly from the change in the number of neutrons, thereby affecting the overall mass of the atom.
Atomic Mass and Average Atomic Mass: Accounting for Isotopic Abundance
The atomic mass listed on the periodic table is not the mass of a single isotope, but rather the average atomic mass, considering the natural abundance of each isotope. This average atomic mass reflects the weighted average of the masses of all isotopes of an element found in nature.
For instance, chlorine (Cl) has two main isotopes: chlorine-35 (³⁵Cl) and chlorine-37 (³⁷Cl). Chlorine-35 is more abundant, making the average atomic mass of chlorine closer to 35 than to 37. The calculation involves multiplying the mass of each isotope by its relative abundance and summing the results.
The proton's contribution remains crucial even in this average calculation. The weighted average still directly reflects the number of protons (and neutrons) present in each isotope, making the proton's mass a foundational aspect in determining the overall atomic mass of an element.
Beyond Mass: The Role of Protons in Atomic Properties
While protons primarily contribute to mass, their role extends far beyond this. The number of protons definitively determines the element's identity and its fundamental chemical properties.
- Chemical behavior: The number of protons dictates the number of electrons in a neutral atom, which then determines the arrangement of electrons in electron shells and orbitals. This electron configuration governs an atom's chemical reactivity and the types of chemical bonds it can form.
- Nuclear stability: The balance between the number of protons and neutrons in the nucleus significantly impacts the stability of the nucleus. Too many or too few neutrons relative to the number of protons can lead to radioactive isotopes that undergo decay.
- Isotopic variations and their impact: The differing numbers of neutrons in isotopes, while not changing the number of protons, can influence various properties such as nuclear stability, radioactive decay rates, and even subtle differences in chemical behavior (isotope effects).
Conclusion: Protons as Fundamental Contributors to Atomic Mass and Properties
In summary, protons are undeniably essential contributors to the mass of an atom. Their positive charge determines the element's identity, while their mass, along with that of the neutrons, accounts for the vast majority of an atom's total mass. Understanding the roles of protons, neutrons, and electrons is fundamental to comprehending atomic structure, chemical properties, and the behavior of matter at the atomic level. The concept of isotopes further emphasizes the direct link between proton number, neutron variability, and the resulting atomic mass, solidifying the proton's crucial position in the atom's properties. The average atomic mass, while taking isotopic abundance into account, ultimately still reflects the inherent contributions of protons and neutrons, highlighting their central role in defining an element's characteristics.
Latest Posts
Latest Posts
-
Difference Between Tlc And Column Chromatography
Mar 15, 2025
-
Energy Required To Remove An Electron From A Gaseous Atom
Mar 15, 2025
-
Que Es La Descomposicion De Acidos
Mar 15, 2025
-
Which Factor Affects Congressional Approval Ratings The Most
Mar 15, 2025
-
Fourier Transform Of A Differential Equation
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Is A Proton Contribute Mass Of An Atom . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
