Is A Single Bond Stronger Than A Triple Bond
Muz Play
Mar 22, 2025 · 6 min read
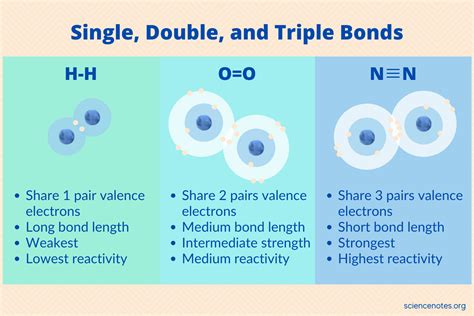
Table of Contents
Is a Single Bond Stronger Than a Triple Bond? Debunking a Common Misconception
The statement "a single bond is stronger than a triple bond" is fundamentally incorrect. In fact, the opposite is true: triple bonds are significantly stronger than single bonds. This misconception often arises from a misunderstanding of the nature of chemical bonding and the interplay between bond strength and other molecular properties. This article will delve deep into the intricacies of single, double, and triple bonds, exploring their strengths, formation, and implications in various chemical contexts.
Understanding Chemical Bonds: The Foundation of Molecular Structure
Before we compare bond strengths, let's establish a clear understanding of what constitutes a chemical bond. A chemical bond is the attractive force that holds atoms together in a molecule. These bonds form because of the electrostatic interaction between the positively charged nuclei of atoms and the negatively charged electrons surrounding them. The most common types of chemical bonds are covalent bonds, where atoms share electrons, and ionic bonds, where atoms transfer electrons. This article primarily focuses on covalent bonds, as they are the context where single, double, and triple bonds are relevant.
Single, Double, and Triple Bonds: A Comparative Analysis
Covalent bonds are formed by the sharing of electron pairs between atoms. A single bond involves the sharing of one electron pair, a double bond involves the sharing of two electron pairs, and a triple bond involves the sharing of three electron pairs. The number of shared electron pairs directly impacts the bond strength and length.
-
Single Bond: Represented as a single line (-) between two atoms. It involves one sigma (σ) bond, which is a strong, localized bond formed by the direct overlap of atomic orbitals.
-
Double Bond: Represented as two lines (=) between two atoms. It comprises one sigma (σ) bond and one pi (π) bond. The pi bond is formed by the sideways overlap of p orbitals, resulting in a weaker bond than the sigma bond.
-
Triple Bond: Represented as three lines (≡) between two atoms. It consists of one sigma (σ) bond and two pi (π) bonds. The presence of two pi bonds further strengthens the bond, but the individual pi bonds are weaker than the sigma bond.
Bond Strength: The Energy Required to Break a Bond
Bond strength is quantified by the bond dissociation energy (BDE), which represents the amount of energy required to break a bond homolytically (cleaving the bond symmetrically, with each atom retaining one electron). Higher BDE values indicate stronger bonds. Since triple bonds involve the sharing of more electron pairs, they have significantly higher BDEs and are thus stronger than double and single bonds.
The key takeaway here is that while individual pi bonds are weaker than sigma bonds, the cumulative effect of multiple bonds (double and triple) results in a stronger overall bond compared to a single bond.
Bond Length: The Distance Between Bonded Atoms
Another crucial aspect to consider is bond length, the average distance between the nuclei of two bonded atoms. As the number of shared electron pairs increases, the bond becomes shorter and stronger. Therefore, triple bonds are shorter than double bonds, which are shorter than single bonds. This shorter distance reflects the stronger electrostatic attraction between the nuclei and the increased electron density in the bonding region.
Examples and Applications: Illustrating the Concepts
Let's consider some real-world examples to illustrate the differences between single, double, and triple bonds and their associated strengths:
-
Ethane (C₂H₆): Contains a single C-C bond. This bond is relatively weak and easily broken.
-
Ethene (C₂H₄): Contains a double C=C bond. The double bond is stronger than the single bond in ethane, making ethene less reactive.
-
Ethyne (C₂H₂): Contains a triple C≡C bond. This triple bond is the strongest of the three, making ethyne the least reactive.
The differences in reactivity are directly related to the bond strengths. The stronger the bond, the more energy is required to break it, resulting in lower reactivity. This is crucial in organic chemistry, where the type of bond significantly influences the chemical behavior of molecules.
Beyond Simple Diatomic Molecules: Complexity in Larger Molecules
While the concepts discussed above are easily illustrated with simple diatomic molecules like N₂, O₂, and others, the situation becomes more complex in larger molecules. The strength of a bond can be influenced by several factors, including:
-
Hybridization: The hybridization of atomic orbitals participating in bond formation influences bond strength and length. For example, sp hybridized orbitals form stronger bonds than sp² or sp³ hybridized orbitals.
-
Resonance: In molecules with delocalized electrons, resonance structures can lead to an average bond order that is not a whole number. This affects the overall bond strength and length.
-
Steric Effects: The spatial arrangement of atoms and substituents can influence bond strength through steric hindrance. Bulky substituents can weaken a bond by increasing steric strain.
-
Inductive Effects: Electron-withdrawing or electron-donating groups can affect the electron density in the bonding region, impacting bond strength.
These factors highlight that while the general rule that triple bonds are stronger than double bonds, which are stronger than single bonds, holds true, the specific strength of a bond in a complex molecule is a nuanced issue dependent on various interacting factors.
Misconceptions and Clarifications: Addressing Common Errors
The misconception that single bonds are stronger than triple bonds likely stems from a misunderstanding of the relationship between bond strength and reactivity. While single bonds are easier to break than triple bonds, this does not imply they are inherently stronger. The ease of breaking a bond depends on the overall stability of the resulting fragments and the activation energy required for bond cleavage.
Another source of confusion may be the different energies required to break a sigma bond versus a pi bond. While pi bonds are weaker individually, their presence alongside a sigma bond in double and triple bonds results in a net stronger bond compared to a single sigma bond.
Conclusion: Triple Bonds Reign Supreme in Bond Strength
In conclusion, the statement "a single bond is stronger than a triple bond" is inaccurate. Triple bonds are demonstrably stronger than double bonds, which are stronger than single bonds. This difference in strength arises from the greater number of shared electron pairs and the resulting stronger electrostatic attraction between the bonded atoms. While other factors can influence bond strength in complex molecules, the fundamental principle remains consistent. Understanding the nuances of chemical bonding, including bond strength and length, is essential for comprehending the reactivity and properties of various molecules. This knowledge is crucial across multiple scientific disciplines, from organic chemistry to materials science and beyond. The strength of a bond, therefore, isn't simply about the number of bonds, but the collective strength of all bonds involved, reflecting the intricate dance of electrons and nuclei that dictates the nature of matter.
Latest Posts
Latest Posts
-
What Is A Factor In An Experiment
Mar 23, 2025
-
Dna Coloring Transcription And Translation Colored
Mar 23, 2025
-
Select The Iupac Name For The Ether
Mar 23, 2025
-
Which Changes In States Have The Most Energy
Mar 23, 2025
-
Chemistry Is The Study Of Matter
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about Is A Single Bond Stronger Than A Triple Bond . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
