Select The Iupac Name For The Ether
Muz Play
Mar 23, 2025 · 5 min read
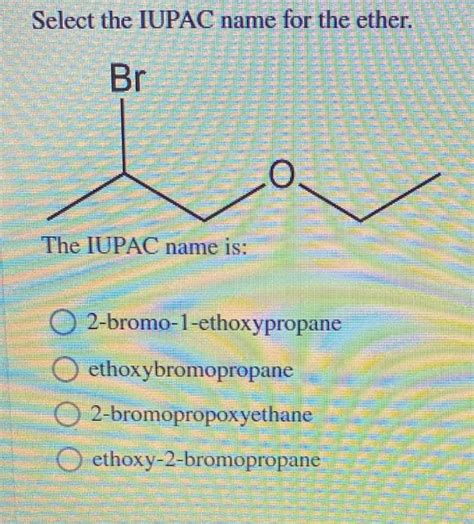
Table of Contents
Selecting the IUPAC Name for an Ether: A Comprehensive Guide
Ethers, a class of organic compounds characterized by an oxygen atom bonded to two alkyl or aryl groups (R-O-R'), form a significant part of organic chemistry. Understanding their nomenclature, particularly according to the International Union of Pure and Applied Chemistry (IUPAC) system, is crucial for accurate communication and effective research in this field. This comprehensive guide will delve into the intricacies of IUPAC ether nomenclature, equipping you with the knowledge to name even the most complex ether structures confidently.
Understanding the Basics of Ether Nomenclature
Before delving into the complexities, let's establish a foundational understanding. The IUPAC system prioritizes a logical and systematic approach, avoiding ambiguity. This contrasts with common names, which can be less precise and often vary regionally. The core principle revolves around identifying the longest carbon chain containing the oxygen atom and treating the shorter alkyl groups as substituents.
Key Steps in IUPAC Ether Nomenclature:
-
Identify the Longest Carbon Chain: This forms the parent alkane. Locate the oxygen atom within this chain.
-
Identify Alkyl Substituents: Determine the alkyl groups attached to the oxygen atom. These are named as alkoxy substituents.
-
Numbering the Carbon Chain: Number the parent chain such that the oxygen atom receives the lowest possible number.
-
Construct the Name: The name begins with the alkoxy substituent(s), followed by the name of the parent alkane. For multiple alkoxy substituents, use prefixes (di-, tri-, tetra-, etc.) and indicate their positions using locants (numbers).
Examples: Simple Ether Nomenclature
Let's illustrate the process with simple examples:
Example 1: CH₃-O-CH₃ (Dimethyl ether)
- Longest Chain: Both chains are equal (one carbon each). We can choose either as the parent chain.
- Alkoxy Substituents: Both are methyl (CH₃) groups.
- Numbering: Not needed in this case as both chains are identical.
- IUPAC Name: Methoxymethane. Note: Common names, like dimethyl ether, are still frequently used, but IUPAC nomenclature provides more precision and avoids ambiguity with other molecules.
Example 2: CH₃-CH₂-O-CH₃ (Methyl ethyl ether)
- Longest Chain: The ethyl group (CH₃CH₂) is the longest chain (two carbons).
- Alkoxy Substituents: One methyl group (CH₃).
- Numbering: The oxygen is attached to carbon 1 of the ethyl group.
- IUPAC Name: Methoxyethane.
Example 3: CH₃-CH₂-O-CH₂-CH₃ (Diethyl ether)
- Longest Chain: Both chains are equal (two carbons each).
- Alkoxy Substituents: Two ethyl groups (CH₃CH₂).
- Numbering: Not needed, as both chains are identical.
- IUPAC Name: Ethoxyethane. This compound is also known by its common name, diethyl ether.
Handling Complex Ether Structures: Advanced Nomenclature
As the complexity of the ether structure increases, so does the intricacy of naming. Let's explore some scenarios requiring a more sophisticated application of IUPAC rules:
Dealing with Branched Alkyl Groups
When branched alkyl groups are present, the IUPAC system prioritizes the longest continuous chain within each alkyl substituent. The substituents on these chains are named accordingly and their positions are indicated by numbers.
Example 4: (CH₃)₂CH-O-CH₂CH₃ (Isopropyl ethyl ether)
- Longest Chains: Isopropyl group [(CH₃)₂CH] and Ethyl group (CH₂CH₃).
- Alkoxy Substituent: 1-methylethoxy
- Numbering: Numbering the ethyl group as the main chain. Oxygen is on carbon 1, the methyl group is on carbon 1.
- IUPAC Name: 1-Methoxyethane (While the common name is isopropyl ethyl ether, IUPAC prioritizes the longest continuous chain, which results in the name above)
Example 5: (CH₃)₂CHOCH(CH₃)₂
- Longest Chains: Both are isopropyl groups
- Alkoxy Substituent: 1-methylethoxy
- Numbering: Identical chains, no need for numbering.
- IUPAC Name: 1-Methoxy-1-methylethane
Ethers with Multiple Alkoxy Substituents
When multiple alkoxy groups are attached to the same carbon atom or different carbon atoms, their positions are precisely indicated using locants. Number prefixes (di-, tri-, tetra-, etc.) are used to denote the number of times a particular alkoxy group appears.
Example 6: CH₃-CH(OCH₃)₂
- Longest Chain: Ethane.
- Alkoxy Substituents: Two methoxy groups.
- Numbering: Both methoxy groups are attached to carbon 2 (if we number from the left).
- IUPAC Name: 2,2-Dimethoxyethane.
Cyclic Ethers (Epoxides and Oxacycloalkanes)
Cyclic ethers, where the oxygen atom is part of a ring, have a specific nomenclature approach. These are often named as oxacycloalkanes, indicating the number of carbon atoms in the ring. Epoxides, three-membered cyclic ethers, are often referred to by their common names or as oxiranes.
Example 7: A three-membered ring with an oxygen and two carbons (Epoxide)
- IUPAC Name: Oxirane (Common names are often used like ethylene oxide)
Example 8: A five-membered ring with an oxygen and four carbons
- IUPAC Name: Oxolane (Tetrahydrofuran is also commonly used)
Example 9: A six-membered ring with an oxygen and five carbons
- IUPAC Name: Oxane (Tetrahydropyran is commonly used)
Applying IUPAC Nomenclature in Research and Practice
The accurate and consistent use of IUPAC nomenclature is crucial in several aspects of organic chemistry:
-
Unambiguous Communication: IUPAC names leave no room for misinterpretations, ensuring clarity in scientific publications, databases, and patents.
-
Accurate Synthesis: Correctly naming the target compound is essential for planning and executing organic syntheses. The IUPAC name guides the choice of starting materials and reaction conditions.
-
Data Management: Chemical databases and software rely on IUPAC nomenclature for efficient organization and retrieval of information.
-
Regulatory Compliance: In fields like pharmaceuticals and agrochemicals, adherence to IUPAC standards is often mandated for regulatory approval.
Conclusion: Mastering the Art of Ether Nomenclature
Mastering IUPAC ether nomenclature is a fundamental skill for any aspiring or practicing organic chemist. Although initially challenging, the systematic approach of IUPAC rules promotes accuracy and consistency in communication. This guide provides a comprehensive overview, covering simple and complex examples to build confidence in applying these rules to a wide range of ether structures. By understanding the fundamental principles, including identifying the longest carbon chain, numbering the chain correctly, naming the alkoxy substituents and considering the presence of branched alkyl groups or cyclic structures, one can effectively and accurately apply IUPAC nomenclature, ensuring clear and unambiguous communication within the field of organic chemistry. Continued practice with diverse ether structures will solidify this important skill, leading to proficiency in naming and understanding this crucial class of organic compounds.
Latest Posts
Latest Posts
-
What Are Rows Called On The Periodic Table
Mar 25, 2025
-
What Group Defines Themselves Through A Rejection Of The Mainstream
Mar 25, 2025
-
Transfer Function Of An Rc Circuit
Mar 25, 2025
-
How Do You Find The Molar Mass Of A Gas
Mar 25, 2025
-
Examples Include Oils Waxes And Butters
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Select The Iupac Name For The Ether . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
