Which Changes In States Have The Most Energy
Muz Play
Mar 23, 2025 · 6 min read
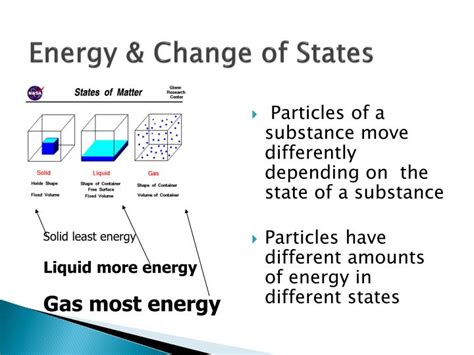
Table of Contents
Which Changes in States Have the Most Energy?
The concept of energy changes during state transitions is fundamental to chemistry and physics. Understanding which changes involve the most energy is crucial in numerous applications, from industrial processes to predicting weather patterns. This comprehensive exploration delves into the energy involved in changes of state, focusing on the relative energy requirements and the underlying principles governing these transformations.
Understanding States of Matter and Their Transitions
Before diving into the energy aspects, let's briefly review the three primary states of matter: solid, liquid, and gas. A solid has a defined shape and volume, with particles closely packed and exhibiting strong intermolecular forces. A liquid has a defined volume but adopts the shape of its container, having weaker intermolecular forces than solids. A gas has neither a defined shape nor volume, with particles widely dispersed and minimal intermolecular interactions. Plasma, a fourth state, exists at extremely high temperatures and involves ionized particles, but we'll primarily focus on the three common states for this discussion.
Transitions between these states – melting (solid to liquid), freezing (liquid to solid), vaporization (liquid to gas), condensation (gas to liquid), sublimation (solid to gas), and deposition (gas to solid) – all involve energy exchange. This energy is often expressed as latent heat, representing the energy absorbed or released during a phase change without altering the temperature.
The Role of Intermolecular Forces
The magnitude of energy change during a state transition is directly related to the strength of intermolecular forces between the constituent particles. Stronger forces require more energy to overcome during transitions like melting or vaporization, while releasing more energy during the reverse transitions.
-
Strong Intermolecular Forces: Substances with strong intermolecular forces (like hydrogen bonding in water) require significantly more energy for transitions involving breaking these bonds. This results in higher latent heats of fusion (melting) and vaporization.
-
Weak Intermolecular Forces: Substances with weak intermolecular forces (like noble gases) require less energy for similar transitions, leading to lower latent heats.
Comparing Energy Changes: A Detailed Analysis
Let's compare the energy changes involved in different state transitions:
1. Vaporization (Liquid to Gas): Typically the Most Energetic
Vaporization, also known as boiling or evaporation, generally involves the most significant energy change. This is because it requires overcoming all intermolecular forces holding the liquid together, completely separating the molecules into the gaseous phase. The energy needed is considerable because molecules must move far apart against attractive forces. The latent heat of vaporization is always significantly higher than the latent heat of fusion for a given substance.
Factors Affecting Vaporization Energy:
-
Strength of Intermolecular Forces: As mentioned, stronger forces lead to higher energy requirements. Water, with its strong hydrogen bonds, has a relatively high latent heat of vaporization.
-
Temperature: Higher temperatures generally lead to easier vaporization, requiring less additional energy input.
-
Pressure: Lower external pressure makes vaporization easier, reducing the energy needed.
2. Fusion (Solid to Liquid): Significant, But Less Than Vaporization
Fusion, or melting, involves the transition from a solid to a liquid. While it requires energy to overcome some intermolecular forces, it doesn't require completely separating the molecules like vaporization does. The molecules gain enough kinetic energy to overcome some of the forces holding them in a fixed structure, allowing them to move more freely, but they are still relatively close together. The energy required for fusion is typically lower than that for vaporization.
Factors Affecting Fusion Energy:
-
Strength of Intermolecular Forces: Stronger forces lead to higher melting points and higher latent heat of fusion.
-
Crystal Structure: The arrangement of particles in the solid influences the energy required to disrupt this structure.
3. Sublimation (Solid to Gas): Moderate Energy Change
Sublimation is the direct transition from a solid to a gas, bypassing the liquid phase. This requires sufficient energy to overcome all intermolecular forces, similar to vaporization, but starting from a more ordered solid state. The energy change in sublimation is generally intermediate between fusion and vaporization, depending on the substance and its properties.
Factors Affecting Sublimation Energy:
-
Strength of Intermolecular Forces: As with other transitions, strong intermolecular forces require more energy for sublimation.
-
Temperature and Pressure: Low pressures and high temperatures favor sublimation.
4. Condensation, Freezing, and Deposition: Exothermic Processes
The reverse transitions – condensation (gas to liquid), freezing (liquid to solid), and deposition (gas to solid) – are all exothermic, releasing energy as intermolecular forces form. The amount of energy released is equal to the energy absorbed during the corresponding endothermic transition (vaporization, fusion, and sublimation, respectively).
Practical Applications and Examples
Understanding these energy changes has numerous practical applications:
-
Cooling Systems: Evaporation of refrigerants in air conditioning and refrigeration units leverages the high latent heat of vaporization to efficiently absorb heat from the surrounding environment.
-
Industrial Processes: Many industrial processes, such as distillation and crystallization, rely on controlled changes of state to separate and purify substances. Knowledge of the energy requirements is crucial for efficient design and operation.
-
Meteorology: Understanding the energy involved in phase transitions of water (evaporation, condensation, etc.) is essential for weather forecasting and climate modeling. Evaporation from oceans and other bodies of water plays a vital role in weather patterns.
-
Material Science: The study of phase transitions is critical for designing materials with specific properties. For example, the melting and solidification behavior of metals is essential for casting and shaping.
Further Considerations: Enthalpy and Entropy
The energy changes during state transitions are closely tied to enthalpy (H) and entropy (S). Enthalpy represents the total heat content of a system, while entropy measures the degree of disorder or randomness.
-
Enthalpy Change (ΔH): The enthalpy change (ΔH) during a phase transition is directly related to the latent heat. For endothermic transitions (melting, vaporization, sublimation), ΔH is positive, indicating an increase in enthalpy. For exothermic transitions, ΔH is negative.
-
Entropy Change (ΔS): Entropy generally increases during phase transitions from solid to liquid and liquid to gas, reflecting an increase in disorder. The entropy change is positive for these transitions.
The relationship between enthalpy, entropy, and temperature (T) is expressed through the Gibbs free energy (G): ΔG = ΔH - TΔS. The spontaneity of a phase transition depends on the sign of ΔG; a negative ΔG indicates a spontaneous process.
Conclusion: A Complex Interplay of Forces and Energy
The energy involved in changes of state is a complex interplay of intermolecular forces, temperature, pressure, and thermodynamic principles. While vaporization typically involves the most significant energy change due to the complete separation of molecules, the energy requirements for other transitions (fusion, sublimation) are still substantial and depend heavily on the specific substance and conditions. A comprehensive understanding of these energy changes is fundamental to various scientific and technological fields. Further research into specific substances and their unique properties will reveal even more nuanced details about the energetic landscape of phase transitions.
Latest Posts
Latest Posts
-
First Formulation Of The Categorical Imperative
Mar 25, 2025
-
Rearrangement Of Benzil To Benzilic Acid
Mar 25, 2025
-
Is Table Salt A Mixture Or Pure Substance
Mar 25, 2025
-
How To Do Inverse Laplace Transforms
Mar 25, 2025
-
Real World Application Of A Linear Equation In 2 Variables
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Which Changes In States Have The Most Energy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
