Is Osmosis High To Low Or Low To High
Muz Play
Mar 26, 2025 · 6 min read
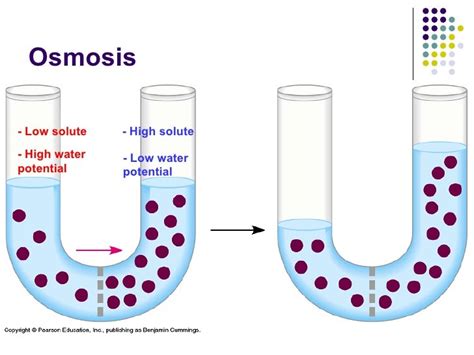
Table of Contents
Is Osmosis High to Low or Low to High? Understanding Osmosis and Osmotic Pressure
Osmosis, a fundamental process in biology and chemistry, often sparks confusion regarding the direction of water movement. Many struggle with the question: does osmosis move from high to low concentration or low to high? The simple answer is high to low water concentration, or equivalently, low to high solute concentration. However, a deeper understanding requires exploring the concepts of water potential, osmotic pressure, and the different types of solutions. This article will delve into these aspects, providing a comprehensive explanation of osmosis and clarifying the seemingly contradictory descriptions.
Understanding the Basics: Water Potential and Solute Concentration
Before tackling the direction of osmosis, let's establish the foundational concepts. Osmosis is the passive movement of water across a selectively permeable membrane from a region of high water potential to a region of low water potential. This movement continues until equilibrium is reached.
-
Water Potential: This term represents the tendency of water to move from one area to another. It's influenced by several factors, but the most significant in osmosis is the solute concentration. Pure water has the highest water potential. Adding solutes lowers the water potential. Think of it like this: the more solutes you add, the less "free" water is available to move.
-
Solute Concentration: This refers to the amount of dissolved substances (solutes) in a given volume of water (solvent). A high solute concentration means there are many solute particles dissolved in the water, and thus, less free water. A low solute concentration means there are fewer solute particles and more free water.
Therefore, water moves from an area with a high concentration of free water molecules (low solute concentration, high water potential) to an area with a lower concentration of free water molecules (high solute concentration, low water potential).
The Role of the Selectively Permeable Membrane
The selectively permeable membrane is crucial to osmosis. This membrane allows the passage of water molecules but restricts the movement of larger solute molecules. This selective permeability creates the driving force for osmosis. Water molecules, being smaller, can easily cross the membrane, while the larger solute molecules cannot. This difference in permeability drives the net movement of water from the area with more free water to the area with less.
Different Types of Solutions and Their Impact on Osmosis
Understanding the different types of solutions helps clarify the direction of water movement in osmosis:
-
Hypotonic Solution: This solution has a lower solute concentration than the solution it's being compared to. Therefore, it has a higher water potential. Water will move into a cell placed in a hypotonic solution.
-
Hypertonic Solution: This solution has a higher solute concentration than the solution it's being compared to. It has a lower water potential. Water will move out of a cell placed in a hypertonic solution.
-
Isotonic Solution: This solution has an equal solute concentration compared to the solution it's being compared to. There's no net movement of water. The water potential is equal on both sides of the membrane.
These solution types further illustrate the concept that water moves from high water potential (hypotonic) to low water potential (hypertonic).
Osmotic Pressure: The Counterforce to Osmosis
Osmotic pressure is the pressure that must be applied to prevent the inward flow of water across a semipermeable membrane. It's a measure of the tendency of water to move into a solution due to the difference in water potential. The higher the solute concentration, the higher the osmotic pressure. This pressure acts as a counterforce to osmosis. If osmotic pressure is high enough, it can prevent any further net movement of water.
Therefore, while water moves from high to low water potential (or low to high solute concentration), the osmotic pressure generated by the solute concentration acts to oppose this movement. The final equilibrium is determined by a balance between the water potential gradient and the osmotic pressure.
Practical Examples of Osmosis
Osmosis is ubiquitous in biological systems. Consider these examples:
-
Plant Cells: Plant cells rely heavily on osmosis to maintain turgor pressure. When placed in a hypotonic solution, water enters the cell, causing it to swell and become turgid. This pressure provides structural support for the plant. In a hypertonic solution, water leaves the cell, leading to plasmolysis (cell shrinkage).
-
Animal Cells: Animal cells also experience changes in volume due to osmosis. In a hypotonic solution, animal cells can lyse (burst) due to excessive water influx. In a hypertonic solution, they crenate (shrink) due to water loss. Maintaining an isotonic environment is crucial for animal cell survival.
-
Water Absorption in Roots: Plants absorb water from the soil through osmosis. The soil solution typically has a lower solute concentration than the root cells, allowing water to move into the plant's roots.
-
Kidney Function: The kidneys regulate water balance in the body by controlling the concentration of solutes in the blood. This control is essential for maintaining the proper water potential in blood and other body fluids.
Misconceptions and Clarifications
It's crucial to address common misconceptions:
-
"Osmosis is low to high concentration": While technically correct if referring to solute concentration, this phrasing can be misleading. It's more accurate and less ambiguous to state that osmosis moves from high to low water potential or high to low water concentration.
-
Ignoring Water Potential: Focusing solely on solute concentration without considering water potential can lead to an incomplete understanding of osmosis. Water potential encompasses all factors influencing water movement, including pressure potential and solute potential.
-
Equilibrium Doesn't Mean No Movement: Even at equilibrium, water molecules still move across the membrane. However, the net movement is zero; the rate of water movement in both directions is equal.
Conclusion: A More Nuanced Understanding
The question of whether osmosis is high to low or low to high requires a nuanced answer. While it's simpler to say "high to low concentration," specifying that it's water concentration moving from high to low or solute concentration moving from low to high offers clarity. A comprehensive understanding requires integrating the concepts of water potential, osmotic pressure, and the different types of solutions. By comprehending these interconnected factors, we can fully grasp the fundamental principles of osmosis and its crucial role in various biological and chemical processes. Remember, the key takeaway is the movement from regions of high water potential (low solute concentration) to regions of low water potential (high solute concentration) until equilibrium is achieved. This understanding is vital in various fields, including biology, medicine, agriculture, and environmental science. The implications of osmotic pressure and water potential are far-reaching, influencing everything from plant growth to human physiology.
Latest Posts
Latest Posts
-
Definition Of Order Of A Reaction
Mar 29, 2025
-
Why Is Blood Clotting A Positive Feedback
Mar 29, 2025
-
What Elements Are Most To Become Anions
Mar 29, 2025
-
Which Place On An Enzyme Binds A Substrate
Mar 29, 2025
-
The Number Of Electrons Moving Is Known As
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Is Osmosis High To Low Or Low To High . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
