Is Salt Water A Mixture Or Compound
Muz Play
Mar 18, 2025 · 6 min read
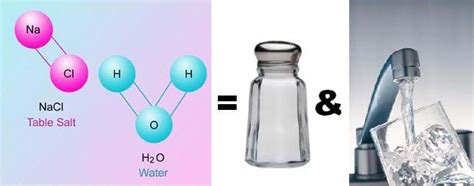
Table of Contents
Is Salt Water a Mixture or a Compound? A Deep Dive into Chemistry
The question, "Is saltwater a mixture or a compound?" seems simple, but delving into the answer reveals a fascinating exploration of chemical concepts fundamental to understanding the world around us. While the concise answer is that saltwater is a mixture, understanding why requires a closer examination of the differences between mixtures and compounds. This exploration will cover the definitions, characteristics, and examples of both mixtures and compounds, emphasizing the specific case of saltwater. We'll also touch upon the implications of this classification in various scientific fields.
Understanding Mixtures and Compounds: The Fundamental Difference
Before classifying saltwater, let's clearly define the terms "mixture" and "compound." This foundational knowledge is crucial for comprehending the nature of saltwater and similar substances.
Mixtures: A Blend of Substances
A mixture is a substance composed of two or more components that are not chemically bonded. The components retain their individual chemical properties and can be physically separated using methods like filtration, distillation, evaporation, or chromatography. Crucially, the composition of a mixture is variable; you can change the proportions of its components without altering the fundamental nature of the mixture itself. Think of a salad—a mixture of lettuce, tomatoes, cucumbers, and dressing. Each ingredient retains its identity, and you can easily adjust the amounts of each.
Key Characteristics of Mixtures:
- Variable composition: The ratio of components can vary.
- Components retain their properties: The individual substances maintain their chemical characteristics.
- Physically separable: Components can be separated using physical methods.
- No chemical reaction occurs: The components don't react chemically to form new substances.
Compounds: Chemically Bonded Substances
A compound, on the other hand, is a substance formed when two or more chemical elements are chemically bonded together. This bonding involves the sharing or transfer of electrons, creating a new substance with properties entirely different from its constituent elements. Compounds have a fixed composition; the ratio of elements is always constant. For example, water (H₂O) is a compound formed from hydrogen and oxygen in a fixed 2:1 ratio. You cannot change this ratio and still have water. Breaking down a compound requires a chemical reaction, not a simple physical separation.
Key Characteristics of Compounds:
- Fixed composition: The ratio of elements is constant.
- New properties emerge: The compound has properties distinct from its constituent elements.
- Chemically separable: Separation requires a chemical reaction.
- Chemical bonds are present: Atoms are linked through chemical bonds.
Saltwater: A Detailed Analysis
Now, let's apply this knowledge to saltwater. Saltwater is essentially a solution of salt (sodium chloride, NaCl) dissolved in water (H₂O). Let's examine this in detail:
-
Salt (NaCl): Sodium chloride is a compound. It's formed from the chemical bonding of sodium (Na) and chlorine (Cl) atoms. These atoms are strongly bonded together, and you can't separate them by simply boiling the salt or filtering it. You need a chemical reaction (like electrolysis) to break it down into its constituent elements.
-
Water (H₂O): As mentioned earlier, water is also a compound, formed from the chemical bonding of hydrogen and oxygen.
-
Saltwater: When salt is dissolved in water, the sodium and chloride ions (Na⁺ and Cl⁻) become dispersed among the water molecules. However, no new chemical bonds are formed between the salt and the water. The salt and water molecules simply mix together. You can easily separate the salt and water through evaporation; the water will evaporate, leaving the salt behind. This clearly demonstrates that saltwater is a mixture.
Types of Mixtures: Understanding the Classification of Saltwater
Mixtures are further classified into homogeneous and heterogeneous mixtures. Understanding this classification provides a more precise understanding of saltwater's nature.
Homogeneous Mixtures: Uniform Composition
A homogeneous mixture has a uniform composition throughout. This means that the components are evenly distributed at a microscopic level, and you can't easily distinguish the individual components with the naked eye. Saltwater is a classic example of a homogeneous mixture. No matter where you take a sample from a saltwater solution, the concentration of salt will be (relatively) the same. Other examples include air and sugar dissolved in water.
Heterogeneous Mixtures: Non-uniform Composition
A heterogeneous mixture has a non-uniform composition. Different parts of the mixture have different properties or compositions. Think of sand and water; you can clearly see the distinct layers of sand and water. Other examples include a salad and soil.
The Importance of Classifying Saltwater as a Mixture
Classifying saltwater as a mixture has significant implications across multiple scientific disciplines:
-
Oceanography: Understanding saltwater as a mixture is crucial for studying ocean currents, salinity gradients, and the distribution of dissolved substances in the ocean. The varying composition of saltwater in different parts of the ocean influences marine life and ecological processes.
-
Chemistry: The properties of saltwater, like its boiling point and freezing point, are different from pure water due to the presence of dissolved salt. This difference is a direct consequence of saltwater being a mixture, with the properties being affected by the proportions of its components.
-
Environmental Science: Studying saltwater as a mixture is essential for understanding the impact of pollution on marine ecosystems. The concentration and distribution of pollutants in saltwater depend on various factors, including the physical and chemical properties of the mixture.
-
Engineering: Engineers need to understand the properties of saltwater when designing structures near the ocean, as its corrosive nature can affect materials. Knowing that saltwater is a mixture allows for accurate predictions of its behavior and its impact on engineering projects.
Further Exploration: Beyond Saltwater
The concept of mixtures and compounds extends far beyond saltwater. Many substances we encounter daily are either mixtures or compounds, or even complex combinations of both. Understanding the distinction helps us appreciate the complexity of the material world. Consider these examples:
-
Air: Air is a homogeneous mixture of various gases, primarily nitrogen and oxygen.
-
Milk: Milk is a heterogeneous mixture containing water, fats, proteins, and carbohydrates.
-
Steel: Steel is an alloy—a mixture of iron and carbon—with enhanced properties compared to pure iron.
-
Sugar (Sucrose): Sugar is a compound, specifically a carbohydrate composed of carbon, hydrogen, and oxygen atoms chemically bonded.
-
Table Salt: As discussed before, table salt (NaCl) is a compound.
Conclusion: A Clear Understanding of Saltwater's Nature
In conclusion, saltwater is undeniably a homogeneous mixture. While its components, salt and water, are themselves compounds, their combination creates a mixture where no new chemical bonds are formed. The individual components retain their properties, and the composition of saltwater is variable. Understanding this fundamental classification is essential for various scientific and engineering applications, from oceanography and chemistry to environmental science and engineering. The ability to differentiate between mixtures and compounds represents a cornerstone of chemical understanding, enabling us to explain a wide range of natural phenomena and technological advancements. The seemingly simple question of whether saltwater is a mixture or compound, therefore, opens a door to a broader understanding of the world's chemical complexity.
Latest Posts
Latest Posts
-
How Many Valence Electrons Does O3 Have
Mar 18, 2025
-
Why Do Solids Have A Definite Shape And Volume
Mar 18, 2025
-
Is Salt Water A Pure Substance
Mar 18, 2025
-
What Are The Functions Of The Family
Mar 18, 2025
-
Trends In The Periodic Table Melting Point
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Is Salt Water A Mixture Or Compound . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
