Johann Wolfgang Döbereiner Contribution To The Periodic Table
Muz Play
Mar 23, 2025 · 6 min read
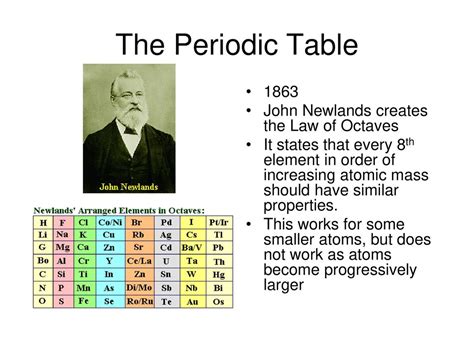
Table of Contents
Johann Wolfgang Döbereiner: A Pioneer in the Quest for Order Among the Elements
Johann Wolfgang Döbereiner, a name less frequently lauded than Mendeleev or Meyer in discussions of the periodic table, nonetheless played a pivotal role in its development. While he didn't create the comprehensive table we use today, his pioneering work in identifying elemental triads laid the crucial groundwork for later advancements. His contributions, though seemingly modest in retrospect, were revolutionary for their time, representing a significant step toward the understanding of the underlying order in the chemical elements. This article will delve into Döbereiner's life, his scientific discoveries, and the lasting impact his triads had on the development of the periodic table.
Döbereiner's Life and Early Work
Born in Hof, Bavaria, on December 13, 1780, Johann Wolfgang Döbereiner's journey to scientific prominence was far from conventional. He was largely self-taught, lacking the formal education that many of his contemporaries enjoyed. His initial training was in apothecary work, which provided a practical foundation in chemistry. This practical experience proved invaluable, grounding his later research in real-world observation and experimentation, rather than solely theoretical pursuits. He later became a professor of chemistry and pharmacy at the University of Jena, a position he held for many years, significantly contributing to the advancement of chemical knowledge in Germany.
His early research covered a wide range of areas within chemistry, including the study of catalysis. This area would ultimately lead him to his most significant contribution to the periodic table. He conducted extensive investigations on various chemical reactions, focusing on the efficiency and mechanisms involved. This meticulous approach to experimental work characterized his scientific style and laid the foundation for his groundbreaking discovery of elemental triads.
The Dawn of Order: Döbereiner's Triads
Döbereiner's most significant contribution to chemistry lies in his discovery of Döbereiner's triads. This was a groundbreaking observation that foreshadowed the periodic law. He noticed that certain groups of three elements (hence, triads) exhibited striking similarities in their properties and that their atomic weights showed a clear pattern. The middle element's atomic weight was approximately the average of the atomic weights of the other two.
Notable Examples of Döbereiner's Triads:
-
The Alkali Metals (Lithium, Sodium, Potassium): Döbereiner observed that the atomic weight of sodium (approximately 23) was roughly the average of lithium (approximately 7) and potassium (approximately 39). This was not merely a coincidence; their chemical properties – all highly reactive metals readily forming +1 ions – also exhibited a strong family resemblance.
-
The Alkaline Earth Metals (Calcium, Strontium, Barium): Similar relationships were observed within this group of elements. The atomic weight of strontium fell between that of calcium and barium, reflecting a parallel trend in their chemical reactivity and the formation of +2 ions.
-
The Halogens (Chlorine, Bromine, Iodine): While not explicitly identified as a triad by Döbereiner himself, this group later proved to fit his observation perfectly, exhibiting a gradual increase in atomic weight and corresponding changes in properties like reactivity.
These observations were significant not only because they pointed toward a pattern among the elements but also because they suggested a fundamental underlying principle governing their properties. This principle wasn't fully understood at the time, but it represented a critical leap forward in the understanding of the chemical world.
Limitations of Döbereiner's Triads
While Döbereiner's triads were an innovative advancement, they had limitations. The system wasn't comprehensive, unable to encompass all known elements. Many elements didn't fit neatly into triads, and several triads displayed exceptions to the averaging rule for atomic weights. These inconsistencies limited the system's applicability and hindered its acceptance as a complete model for organizing the elements.
Furthermore, Döbereiner’s understanding of the underlying cause of these relationships remained somewhat limited. He correctly noted patterns, but he couldn't explain why these patterns existed, a crucial missing link that later scientists would address. The absence of a robust theoretical framework underpinning his observations prevented his work from being universally embraced.
Döbereiner's Other Contributions
Beyond his famous triads, Döbereiner made other significant contributions to chemistry. He is also well-known for inventing the Döbereiner's lamp, a fascinating device that utilized a catalytic reaction to produce a flame. This ingenious invention demonstrated his practical skills and underscored his understanding of catalysis.
This lamp, featuring a piece of platinum acting as a catalyst, ignited hydrogen gas in contact with air, providing a reliable, easily-produced flame. While not directly related to the periodic table, this invention showcased Döbereiner’s innovative spirit and experimental prowess.
The Legacy of Döbereiner's Work
Despite the limitations of his triads, Döbereiner's work laid essential groundwork for the development of the periodic table. His discovery demonstrated that elements were not merely a random collection but exhibited predictable relationships based on their atomic weights and properties. This concept of recurring patterns was crucial to the later development of the periodic law. His work inspired other scientists to seek a more comprehensive system for classifying the elements.
Scientists like Alexandre-Émile Béguyer de Chancourtois, John Newlands, and Dmitri Mendeleev built upon Döbereiner’s foundation. De Chancourtois’ telluric helix, Newlands’ law of octaves, and ultimately Mendeleev’s periodic table were all steps forward in constructing a more complete and systematic organization of the elements, heavily influenced by Döbereiner's initial foray into recognizing elemental relationships.
Döbereiner's Place in the History of Chemistry
Johann Wolfgang Döbereiner's place in the history of chemistry is secure, even if his name isn't as widely recognized as some of his successors. He was a pioneer, a self-taught scientist who made a crucial contribution to one of the most important concepts in chemistry: the periodic table. His discovery of elemental triads, while imperfect, demonstrated the existence of underlying order within the seemingly chaotic collection of chemical elements. His work inspired future generations of scientists, directly paving the way for the development of a more complete and comprehensive system of organization. His legacy lies not just in his specific discoveries, but also in his demonstration that seemingly disparate elements could be linked through systematic observation and careful analysis, a foundation that underscores all of modern chemistry.
His meticulous experimental work, his innovative spirit, and his relentless pursuit of understanding the natural world are testaments to his scientific legacy. Döbereiner's contributions serve as a reminder that even seemingly incremental advancements can have a profound and lasting impact on the course of scientific discovery. His story is a testament to the power of observation, the importance of perseverance, and the enduring quest for order in the universe. He stands as an unsung hero whose early work proved essential to the development of one of science's most elegant and powerful organizational tools: the periodic table.
Latest Posts
Latest Posts
-
What Is The Horizontal Rows On The Periodic Table Called
Mar 24, 2025
-
Understanding The Difference Between Strong And Weak Acids
Mar 24, 2025
-
What Are The 3 Parts Of Atp
Mar 24, 2025
-
Does Covalent Compounds Dissolve In Water
Mar 24, 2025
-
What Color Does Magnesium Metal Burn
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about Johann Wolfgang Döbereiner Contribution To The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
