Lanthanides And Actinides On The Periodic Table
Muz Play
Mar 23, 2025 · 6 min read
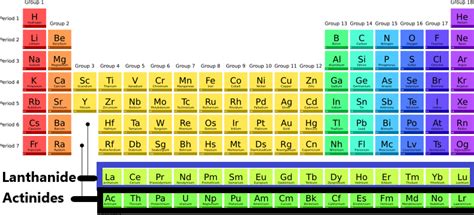
Table of Contents
Lanthanides and Actinides: The Inner Secrets of the Periodic Table
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. While the main group elements (s- and p-block) are familiar to many, the f-block elements—the lanthanides and actinides—often remain shrouded in mystery. This comprehensive guide delves into the fascinating world of these inner transition metals, exploring their unique characteristics, applications, and the challenges associated with their study.
Understanding the f-Block: Lanthanides and Actinides
The lanthanides and actinides, collectively known as the inner transition metals or f-block elements, occupy the two rows positioned below the main body of the periodic table. This placement reflects their electronic configuration, where the 4f and 5f orbitals are progressively filled, respectively. This filling of inner orbitals dictates their similar chemical properties and creates unique challenges in their separation and study.
The Lanthanides: The Rare Earth Elements
The lanthanides, encompassing elements 57 (Lanthanum) through 71 (Lutetium), are often referred to as rare earth elements (REEs). While the term "rare" suggests scarcity, this is a misnomer. Many lanthanides are relatively abundant in the Earth's crust, but their geochemical behavior results in their dispersed distribution, making extraction and purification more challenging and costly.
Key Characteristics of Lanthanides:
- Similar Chemical Properties: Due to the similar electronic configurations and shielding effect of the 4f electrons, lanthanides exhibit remarkably similar chemical properties. This similarity makes their separation and purification a complex process. Techniques like ion exchange chromatography and solvent extraction are employed to achieve effective separation.
- Variable Oxidation States: While the +3 oxidation state is most common, some lanthanides can also exist in +2 or +4 states, although these are less stable. This variability influences their reactivity and the types of compounds they form.
- Magnetic Properties: Many lanthanides exhibit strong paramagnetic properties due to the unpaired electrons in their 4f orbitals. This characteristic is exploited in various applications, such as in permanent magnets and magnetic resonance imaging (MRI) contrast agents.
- Luminescent Properties: Certain lanthanides exhibit bright luminescence when excited by light or other forms of energy. This property is utilized in various applications, including lighting, displays, and lasers. For example, europium is used in red phosphors in color televisions and terbium in green phosphors.
- Catalytic Activity: Several lanthanides and their compounds demonstrate significant catalytic activity. They are employed as catalysts in various industrial processes, including petroleum refining and the production of plastics.
Applications of Lanthanides:
The unique properties of lanthanides have led to their widespread use in a diverse range of applications, including:
- Magnets: Neodymium-iron-boron (NdFeB) magnets, incorporating neodymium, are incredibly powerful permanent magnets used in various applications, such as wind turbines, electric motors, and hard disk drives. Samarium-cobalt (SmCo) magnets offer superior temperature resistance.
- Lighting and Displays: Lanthanides are crucial components in fluorescent lamps, LEDs, and LCD screens. Their luminescent properties provide vibrant and energy-efficient light sources.
- Catalysis: Lanthanides are used as catalysts in various industrial processes, such as petroleum cracking and polymerization.
- Medical Applications: Certain lanthanides are used as contrast agents in MRI scans, enhancing the visibility of organs and tissues. They are also explored for their potential in cancer therapy.
- Ceramics and Glass: Lanthanides are added to ceramics and glass to enhance their properties, such as strength, color, and refractive index. For instance, cerium oxide is a significant polishing agent.
The Actinides: The Radioactive Series
The actinides, encompassing elements 89 (Actinium) through 103 (Lawrencium), are all radioactive. Their radioactivity stems from the instability of their heavy nuclei, leading to spontaneous decay. This inherent radioactivity necessitates specialized handling and safety precautions.
Key Characteristics of Actinides:
- Radioactivity: All actinides are radioactive, exhibiting varying degrees of radioactivity and different decay modes (alpha, beta, gamma). Their half-lives range from fractions of a second to millions of years.
- Complex Chemistry: The actinides exhibit complex and diverse oxidation states, unlike the lanthanides. This is attributed to the less effective shielding of the 5f electrons compared to the 4f electrons in the lanthanides. Common oxidation states include +3, +4, +5, and +6.
- Metallic Properties: Similar to the lanthanides, actinides are generally metallic, exhibiting metallic luster and conductivity.
- Reactivity: Actinides are highly reactive, readily reacting with oxygen, halogens, and acids.
- Nuclear Properties: Their primary importance lies in their nuclear properties. Many actinides are used as nuclear fuels, in nuclear weapons, and in various applications involving nuclear technology.
Applications of Actinides:
The applications of actinides are primarily centered around their nuclear properties:
- Nuclear Fuel: Uranium-235 and Plutonium-239 are the most significant actinides used as nuclear fuels in nuclear power plants. They undergo fission, releasing enormous amounts of energy.
- Nuclear Weapons: Plutonium-239 plays a crucial role in the design of nuclear weapons.
- Nuclear Medicine: Some actinides, like Americium-241, find use in smoke detectors due to their alpha decay properties.
- Research and Scientific Applications: Actinides are used extensively in scientific research, particularly in nuclear physics and chemistry.
Challenges in Studying Lanthanides and Actinides
Despite their importance, studying and working with lanthanides and actinides present several unique challenges:
- Separation Difficulties: The similar chemical properties of lanthanides, in particular, make their separation and purification a difficult and energy-intensive process. Advanced separation techniques are required.
- Radioactivity: The radioactivity of actinides presents significant health and safety hazards, necessitating specialized handling procedures and equipment.
- Toxicity: Some lanthanides and actinides can be toxic, requiring careful handling and disposal procedures.
- Cost: The extraction and purification of lanthanides and actinides can be expensive, limiting their widespread use in certain applications.
The Future of Lanthanides and Actinides Research
Research into lanthanides and actinides continues to be a vibrant area of scientific inquiry. Ongoing efforts focus on:
- Developing more efficient and sustainable separation techniques: Reducing the energy consumption and environmental impact of separating lanthanides is a key focus.
- Exploring new applications: Researchers are actively seeking new and innovative applications for both lanthanides and actinides. This includes applications in renewable energy, advanced materials, and medicine.
- Understanding fundamental properties: A deeper understanding of the electronic structures and chemical behavior of these elements is crucial for developing new applications and improving existing ones.
- Managing radioactive waste: Developing effective strategies for the safe management and disposal of radioactive waste from actinide-based applications is critical for environmental sustainability.
Conclusion: Unlocking the Potential of the Inner Transition Metals
The lanthanides and actinides, though often overlooked, represent a significant portion of the periodic table's richness and complexity. Their unique properties, from luminescence to radioactivity, have already yielded numerous technological advancements. Continued research and innovation promise to unlock even more potential, driving further breakthroughs in various fields, from renewable energy and advanced materials to medical applications and environmental remediation. The challenges associated with their study are substantial, but the potential rewards make the effort worthwhile. Understanding and harnessing the capabilities of these inner transition metals is crucial for future technological advancements and a more sustainable future.
Latest Posts
Latest Posts
-
Group 5a On The Periodic Table
Mar 25, 2025
-
A Neutron Has Approximately The Same Mass As
Mar 25, 2025
-
Atomic Mass Unit Vs Molar Mass
Mar 25, 2025
-
Concept Map Body Cavities And Membranes
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Lanthanides And Actinides On The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
