Matrix Assisted Laser Desorption Ionization Maldi
Muz Play
Mar 24, 2025 · 7 min read
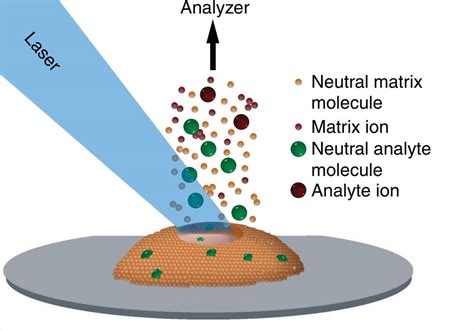
Table of Contents
Matrix-Assisted Laser Desorption/Ionization (MALDI): A Deep Dive into the Technique
Matrix-Assisted Laser Desorption/Ionization (MALDI) is a powerful and versatile ionization technique predominantly used in mass spectrometry (MS) for analyzing large biomolecules such as proteins, peptides, and polymers. Unlike other ionization methods, MALDI's soft ionization process minimizes fragmentation, allowing for the determination of the intact molecular weight of these complex molecules. This article provides a comprehensive overview of MALDI, covering its principles, instrumentation, applications, advantages, and limitations.
Understanding the Fundamentals of MALDI
MALDI's success lies in its unique approach to ionizing large molecules. The process involves two key components:
1. The Matrix: A small organic molecule, typically a weak organic acid, that absorbs the laser energy. The matrix is chosen based on its compatibility with the analyte and its ability to efficiently transfer energy to the analyte without causing excessive fragmentation. Common matrices include α-cyano-4-hydroxycinnamic acid (CHCA), sinapinic acid, and 2,5-dihydroxybenzoic acid (DHB).
2. The Analyte: The biomolecule of interest that needs to be analyzed. The analyte is mixed with the matrix to form a co-crystallized mixture.
The process unfolds as follows:
- Co-crystallization: The matrix and analyte are mixed in a suitable solvent and allowed to co-crystallize, typically by air-drying on a sample plate. This process ensures that the analyte molecules are embedded within the matrix crystals.
- Laser Irradiation: A pulsed laser beam, often a nitrogen laser (337 nm), irradiates the sample. The matrix absorbs the laser energy, leading to its rapid heating and desorption.
- Desorption and Ionization: The energy absorbed by the matrix is transferred to the analyte molecules, causing them to desorb from the surface as gas-phase ions. The ionization mechanism is complex and not fully understood, but it involves proton transfer from the matrix to the analyte, resulting in the formation of predominantly singly charged ions.
- Mass Analysis: The ions are then accelerated and separated based on their mass-to-charge ratio (m/z) in a mass spectrometer, generating a mass spectrum.
The Magic Behind the Soft Ionization: A Closer Look
The "soft" ionization nature of MALDI is crucial for analyzing large biomolecules. Harsh ionization techniques often fragment the analyte, making it difficult to determine its intact molecular weight. MALDI minimizes fragmentation because the energy absorbed by the matrix is distributed efficiently, preventing excessive energy from reaching the analyte. This results in the production of predominantly singly charged molecular ions ([M+H]+ or [M-H]−), facilitating accurate mass determination.
MALDI Instrumentation: A Detailed Overview
A typical MALDI-TOF MS system consists of the following components:
1. Sample Preparation Station: This is where the matrix and analyte are mixed and co-crystallized onto a target plate. Precise sample preparation is critical for achieving high-quality mass spectra.
2. MALDI Ion Source: This houses the target plate and the laser. The laser irradiates the sample, leading to desorption and ionization. Precise control of the laser parameters, such as pulse energy and wavelength, is essential for optimizing the ionization process.
3. Mass Analyzer: The most common mass analyzer used in MALDI-MS is the time-of-flight (TOF) mass analyzer. In a TOF analyzer, ions are accelerated by an electric field and travel through a field-free drift tube. The time it takes for an ion to reach the detector is directly proportional to its mass-to-charge ratio. This allows for the separation and detection of ions based on their m/z values. Other mass analyzers, such as ion traps and orbitraps, can also be coupled with MALDI.
4. Detector: The detector measures the arrival time of the ions and converts this information into a mass spectrum. Common detectors include microchannel plates and electron multipliers.
5. Data Acquisition System: This system controls the instrument's operation, acquires the data from the detector, and processes the mass spectral data.
Applications of MALDI: A Wide Spectrum of Possibilities
MALDI-MS has found widespread applications across diverse scientific fields, including:
1. Proteomics: MALDI-MS is a cornerstone technique in proteomics, the large-scale study of proteins. It is used for protein identification, quantification, and characterization. This includes the determination of protein molecular weights, post-translational modifications, and protein-protein interactions.
2. Glycomics: The study of glycans (carbohydrates) benefits greatly from MALDI's ability to analyze complex carbohydrate structures. MALDI-MS helps in determining glycan structures, identifying glycoproteins, and understanding the roles of glycans in biological processes.
3. Metabolomics: MALDI-MS is instrumental in metabolomics, the study of small molecules (metabolites) in biological systems. It facilitates the identification and quantification of metabolites, aiding in understanding metabolic pathways and disease processes.
4. Polymer Chemistry: MALDI-MS is used for characterizing the molecular weight distribution, end groups, and composition of synthetic polymers. This is particularly valuable in polymer synthesis and materials science.
5. Clinical Diagnostics: MALDI-MS is increasingly used in clinical diagnostics for various applications, including biomarker discovery, infectious disease diagnosis, and drug development.
6. Forensic Science: MALDI-MS finds applications in forensic science for the identification of drugs, explosives, and other forensic samples.
Advantages of MALDI-MS: Why It Stands Out
Several factors contribute to MALDI's widespread adoption:
- High Sensitivity: MALDI-MS boasts high sensitivity, enabling the detection of even femtomole quantities of analytes.
- Soft Ionization: The soft ionization process minimizes fragmentation, making it ideal for analyzing large and fragile biomolecules.
- Ease of Use: MALDI is relatively easy to use compared to other ionization techniques.
- Versatility: It can analyze a wide range of biomolecules, including proteins, peptides, lipids, carbohydrates, and polymers.
- High Throughput: MALDI can be automated for high-throughput analysis, facilitating large-scale studies.
Limitations of MALDI-MS: Areas for Improvement
Despite its advantages, MALDI-MS has some limitations:
- Matrix Interference: The matrix can sometimes interfere with the analysis, leading to background noise and signal suppression.
- Salt Effects: High salt concentrations in samples can suppress ionization and reduce the quality of the mass spectra.
- Reproducibility: Reproducibility can be challenging due to variations in sample preparation and laser parameters.
- Quantitative Analysis: While MALDI can be used for quantitative analysis, it is not as precise as some other techniques.
- Limited Applicability to Certain Analytes: Some analytes may not be amenable to MALDI analysis due to their chemical properties or inability to co-crystallize with the matrix.
Future Directions: The Evolution of MALDI
Research continues to refine and extend the capabilities of MALDI-MS. Areas of ongoing development include:
- Improved Matrix Development: The development of new matrices with improved properties, such as enhanced sensitivity and reduced interference, remains an active area of research.
- Advanced Mass Analyzers: Coupling MALDI with advanced mass analyzers, such as ion traps and orbitraps, enhances the sensitivity, resolution, and versatility of the technique.
- Automation and High-Throughput Screening: Automation and miniaturization are continually improving the throughput and efficiency of MALDI-MS.
- Imaging Mass Spectrometry: MALDI imaging is becoming increasingly important, providing spatial information about the distribution of molecules within tissues and cells.
- Improved Data Analysis Software: Advanced data analysis software helps to improve data interpretation and extract meaningful biological information from the complex mass spectra.
Conclusion: The Undisputed Powerhouse of Biomolecular Analysis
MALDI-MS remains a powerful and versatile technique for analyzing a wide range of biomolecules. Its soft ionization, high sensitivity, and ease of use have made it indispensable in various scientific fields. Ongoing improvements and advancements continue to expand its capabilities and applications, solidifying its position as a cornerstone technique in biomolecular analysis for years to come. The continuous development in matrix design, coupled with advanced mass analyzer technologies, promises even greater sensitivity, accuracy, and high-throughput capabilities, pushing the boundaries of biological and chemical research. Further improvements in software and data analysis techniques will also play a crucial role in unlocking the full potential of MALDI, allowing researchers to glean even deeper insights into complex biological systems.
Latest Posts
Latest Posts
-
How To Calculate The Expected Frequency
Mar 26, 2025
-
Positive And Increasing Rate Of Change
Mar 26, 2025
-
Anything That Has Mass And Volume Is Called
Mar 26, 2025
-
The Diels Alder Reaction Is A Concerted Reaction Define Concerted
Mar 26, 2025
-
How Many Fatty Acids Are Needed To Form A Glycerophospholipid
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Matrix Assisted Laser Desorption Ionization Maldi . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
