Anything That Has Mass And Volume Is Called
Muz Play
Mar 26, 2025 · 6 min read
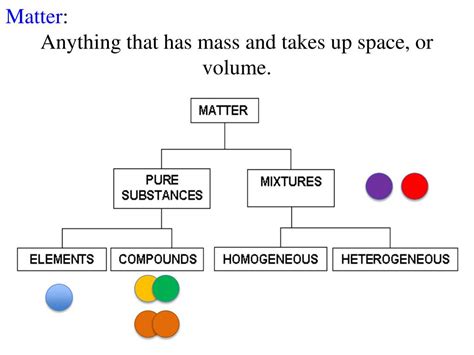
Table of Contents
Anything That Has Mass and Volume Is Called: Exploring Matter and Its Properties
Have you ever stopped to consider what makes up the world around us? Everything we see, touch, and interact with – from the vast expanse of the ocean to the tiniest grain of sand – shares a fundamental characteristic: it possesses both mass and volume. Anything that possesses these two properties is classified as matter. This seemingly simple definition opens the door to a fascinating exploration of physics, chemistry, and the very fabric of our universe. Let's delve deeper into the concepts of mass and volume and how they define matter.
Understanding Mass: A Measure of Inertia
Mass, in its simplest form, is a measure of an object's inertia. Inertia is the resistance an object exhibits to changes in its state of motion. A more massive object requires a greater force to accelerate it to the same extent as a less massive object. Think of pushing a shopping cart versus pushing a loaded truck; the truck, having significantly more mass, is much harder to move.
Mass is often confused with weight, but they are distinct concepts. Weight is the force of gravity acting on an object's mass. Your weight can change depending on your location (e.g., on the moon, your weight would be less than on Earth), but your mass remains constant. Mass is an intrinsic property of an object, while weight is dependent on external forces. We measure mass in units like kilograms (kg) and grams (g).
Types of Mass: Inertial and Gravitational
While the everyday understanding of mass suffices for many applications, physicists distinguish between two types:
-
Inertial Mass: This refers to an object's resistance to acceleration, as described above. It's the mass we experience when we try to push or pull an object.
-
Gravitational Mass: This refers to the strength with which an object attracts other objects due to gravity. It's the mass that determines the gravitational force between two objects, as described by Newton's Law of Universal Gravitation.
Einstein's theory of general relativity elegantly connects these two types of mass, demonstrating their equivalence.
Understanding Volume: A Measure of Space
Volume, on the other hand, is a measure of the three-dimensional space occupied by an object. It quantifies how much space an object takes up. We can visualize volume as the amount of water displaced when an object is submerged in a container.
The units used to measure volume are cubic meters (m³), cubic centimeters (cm³), and liters (L), among others. For irregularly shaped objects, determining volume can be more challenging and might involve techniques like water displacement. However, for regularly shaped objects like cubes and spheres, calculating volume is a straightforward mathematical process based on their dimensions.
Calculating Volume for Different Shapes
The formula for calculating volume depends on the object's shape:
- Cube: Volume = side × side × side = side³
- Rectangular Prism: Volume = length × width × height
- Sphere: Volume = (4/3)πr³ (where r is the radius)
- Cylinder: Volume = πr²h (where r is the radius and h is the height)
The Interplay of Mass and Volume: Density
The relationship between mass and volume gives rise to another crucial property of matter: density. Density is defined as the mass per unit volume of a substance. It tells us how much mass is packed into a given amount of space. A high-density material, like gold, has a large amount of mass concentrated in a small volume, while a low-density material, like air, has a small amount of mass spread over a large volume.
The formula for density is:
Density = Mass / Volume
Density is typically expressed in units like kg/m³ or g/cm³. Understanding density is critical in various applications, from determining the buoyancy of objects to identifying unknown substances.
Applications of Density
The concept of density has numerous applications across diverse fields:
-
Material Science: Density is a crucial factor in selecting materials for various applications. High-density materials are often chosen for strength and durability, while low-density materials are preferred for lightweight applications like aerospace engineering.
-
Geology: Density differences between rocks and minerals help geologists understand the Earth's structure and composition. Seismic waves travel at different speeds through materials of varying densities, providing valuable information about the Earth's interior.
-
Oceanography: Density variations in seawater, driven by temperature and salinity, create ocean currents that play a critical role in global climate regulation.
-
Fluid Mechanics: Density is a fundamental parameter in fluid mechanics, influencing the behavior of liquids and gases in various contexts, such as pipe flow and aerodynamic design.
States of Matter: Solid, Liquid, and Gas
Matter exists in various states, primarily solid, liquid, and gas. These states are characterized by the arrangement and interaction of their constituent particles (atoms and molecules). The differences in the states of matter are directly related to the strength of the intermolecular forces and the kinetic energy of the particles.
-
Solids: In solids, particles are tightly packed and have strong intermolecular forces. They have a fixed shape and volume and are relatively incompressible. Examples include ice, rocks, and metals.
-
Liquids: In liquids, particles are closer together than in gases but further apart than in solids. They have weaker intermolecular forces compared to solids. Liquids have a fixed volume but can adapt to the shape of their container. Examples include water, oil, and mercury.
-
Gases: In gases, particles are widely dispersed and have weak intermolecular forces. They have neither a fixed shape nor a fixed volume and are highly compressible. Examples include air, oxygen, and carbon dioxide.
Plasma: The Fourth State of Matter
Beyond the three common states, there's a fourth state of matter called plasma. Plasma is an ionized gas where a significant portion of the atoms have lost or gained electrons, creating a mixture of ions and free electrons. Plasma is often found in high-temperature environments, such as stars and lightning.
Beyond Mass and Volume: Other Properties of Matter
While mass and volume are fundamental properties defining matter, several other properties help characterize and distinguish different types of matter:
-
Chemical Properties: These describe how a substance reacts with other substances. Examples include flammability, reactivity with acids, and toxicity.
-
Physical Properties: These can be observed or measured without changing the substance's chemical composition. Examples include color, odor, melting point, boiling point, and conductivity.
-
Extensive Properties: These depend on the amount of matter present. Examples include mass, volume, and length.
-
Intensive Properties: These do not depend on the amount of matter present. Examples include density, temperature, and boiling point.
Conclusion: Matter, the Building Block of the Universe
Anything that has mass and volume is undeniably matter, the fundamental building block of our universe. Understanding the concepts of mass, volume, density, and the various states of matter provides a crucial foundation for comprehending the physical world around us. From the smallest atom to the largest galaxy, matter's properties and interactions shape the universe as we know it. The continued exploration of matter's intricacies continues to unlock new scientific breakthroughs and technological advancements. As we deepen our understanding of matter's fundamental properties, we unlock a deeper appreciation for the complexity and beauty of our universe. The study of matter continues to be a vibrant and ever-evolving field, pushing the boundaries of scientific knowledge and technological innovation. This exploration promises to yield even more fascinating discoveries in the years to come.
Latest Posts
Latest Posts
-
Writing Geometric Series In Summation Notation
Mar 26, 2025
-
Is Osmosis High To Low Or Low To High
Mar 26, 2025
-
What Is The Electron Configuration For Cobalt
Mar 26, 2025
-
How Do You Calculate The Average Acceleration
Mar 26, 2025
-
What Subatomic Particles Make Up An Atom
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Anything That Has Mass And Volume Is Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
