What Is The Electron Configuration For Cobalt
Muz Play
Mar 26, 2025 · 5 min read
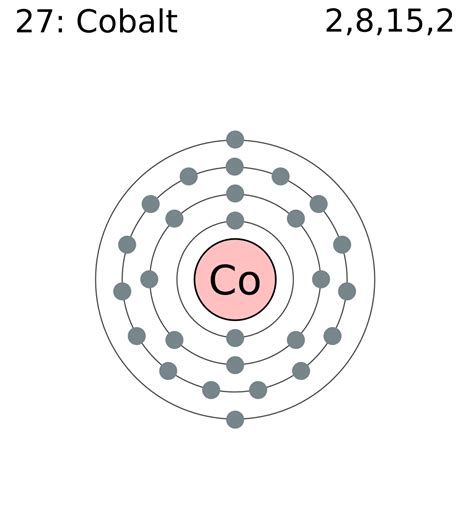
Table of Contents
What is the Electron Configuration for Cobalt? A Deep Dive into Atomic Structure
Cobalt, a transition metal with the symbol Co and atomic number 27, holds a fascinating place in the periodic table. Understanding its electron configuration is key to unlocking its unique chemical and physical properties. This comprehensive guide will delve into the electron configuration of cobalt, exploring its implications for its reactivity, magnetic behavior, and applications in various fields.
Understanding Electron Configuration
Before diving into the specifics of cobalt, let's establish a foundational understanding of electron configuration. The electron configuration of an atom describes how electrons are distributed among its various energy levels and sublevels. These energy levels are represented by principal quantum numbers (n = 1, 2, 3, etc.), and within each level are sublevels designated by s, p, d, and f orbitals. Each orbital can hold a maximum of two electrons, according to the Pauli Exclusion Principle. The Aufbau principle dictates that electrons fill the lowest energy levels first, and Hund's rule specifies that electrons fill orbitals individually before pairing up.
These principles are crucial for predicting the chemical behavior of an element. The outermost electrons, known as valence electrons, are particularly important because they participate in chemical bonding. The arrangement of these valence electrons determines an element's reactivity and the types of compounds it can form.
Determining Cobalt's Electron Configuration
Cobalt has an atomic number of 27, meaning it possesses 27 protons and, in its neutral state, 27 electrons. To determine its electron configuration, we follow the Aufbau principle:
-
The first energy level (n=1): This level contains only the 1s sublevel, which can hold a maximum of two electrons. Therefore, we fill it completely: 1s².
-
The second energy level (n=2): This level contains the 2s and 2p sublevels. The 2s sublevel holds two electrons (2s²), and the 2p sublevel holds six (2p⁶). In total, the second energy level is filled with eight electrons.
-
The third energy level (n=3): This level holds the 3s, 3p, and 3d sublevels. The 3s sublevel holds two electrons (3s²), and the 3p sublevel holds six (3p⁶).
-
The fourth energy level (n=4): This is where things get interesting for cobalt. The 4s sublevel fills first, accommodating two electrons (4s²). After the 4s sublevel is complete, we move to the 3d sublevel. Since cobalt has 27 electrons, and we've already accounted for 20 (2 + 8 + 2 + 6 + 2), we have 7 electrons remaining. These seven electrons fill the 3d sublevel, resulting in a 3d⁷ configuration.
Therefore, the full electron configuration for cobalt is: 1s²2s²2p⁶3s²3p⁶4s²3d⁷.
Orbital Diagrams and Hund's Rule
While the electron configuration provides a concise summary of electron distribution, orbital diagrams offer a more detailed visualization. These diagrams show each orbital as a box, with electrons represented as arrows. Hund's rule states that electrons will individually occupy each orbital within a subshell before pairing up. This is because electrons repel each other, and occupying separate orbitals minimizes this repulsion.
For cobalt's 3d⁷ configuration, the orbital diagram would show seven electrons distributed across the five 3d orbitals, with three orbitals singly occupied and two orbitals doubly occupied. This arrangement maximizes the total spin of the atom, resulting in its paramagnetic behavior.
Visual Representation (Simplified):
1s: ↑↓
2s: ↑↓
2p: ↑↓ ↑↓ ↑↓
3s: ↑↓
3p: ↑↓ ↑↓ ↑↓
4s: ↑↓
3d: ↑ ↑ ↑ ↑ ↑ ↓ ↓
Cobalt's Properties and Electron Configuration
Cobalt's electron configuration is directly responsible for its properties:
Magnetic Properties:
The partially filled 3d orbitals are the primary reason cobalt is a ferromagnetic material. Ferromagnetism arises from the parallel alignment of electron spins in multiple atoms, creating strong magnetic domains. This property makes cobalt highly valuable in the production of magnets and magnetic recording media.
Chemical Reactivity:
Cobalt's valence electrons (4s² and 3d⁷) participate in chemical bonding. It exhibits variable oxidation states, commonly +2 and +3, due to the ease with which it can lose electrons from its outermost 4s and 3d orbitals. This versatility contributes to the formation of numerous cobalt compounds with diverse applications.
Catalytic Activity:
Cobalt's ability to readily change its oxidation state makes it an effective catalyst in various chemical reactions. For example, cobalt compounds are used in the synthesis of ammonia and in hydroformylation processes, vital reactions in industrial chemistry.
Cobalt's Applications: A Reflection of its Electronic Structure
The unique properties stemming from its electron configuration have made cobalt indispensable across numerous applications:
-
Magnets: Cobalt's ferromagnetism contributes to the production of powerful and durable magnets used in various devices, from electric motors and generators to hard disk drives and medical imaging equipment. Alnico magnets, which contain cobalt, are known for their exceptional magnetic strength and temperature stability.
-
Alloys: Cobalt is added to alloys to enhance their strength, hardness, and corrosion resistance. Steels containing cobalt exhibit exceptional wear resistance and are used in high-speed cutting tools and gas turbine blades. Superalloys, incorporating cobalt, nickel, and other elements, withstand extreme temperatures and are critical components in jet engines.
-
Catalysis: Cobalt's catalytic activity is utilized in various industrial processes. Cobalt catalysts are employed in the production of chemicals, including alcohols, aldehydes, and other essential compounds.
-
Pigments: Cobalt compounds contribute to the vivid colors of various pigments used in paints, ceramics, and glass. Cobalt blue, a deep and intense blue pigment, is widely used in artistic and industrial applications.
-
Biomedical Applications: Cobalt-60, a radioactive isotope of cobalt, is used in radiotherapy for cancer treatment. Its gamma radiation is effective in destroying cancerous cells.
-
Batteries: Cobalt is a key component in lithium-ion batteries, powering many electronic devices and electric vehicles. This application emphasizes the importance of cobalt in our transition to renewable energy technologies.
Conclusion: The Significance of Cobalt's Electron Configuration
The electron configuration of cobalt, 1s²2s²2p⁶3s²3p⁶4s²3d⁷, is fundamental to understanding its unique properties and diverse applications. Its partially filled 3d orbitals are responsible for its ferromagnetism, variable oxidation states, and catalytic activity. These properties are integral to cobalt's role in various industries, from the production of powerful magnets to the development of life-saving medical treatments and environmentally friendly energy solutions. Further research into cobalt's behavior continues to uncover new applications and advance technological innovation, solidifying its importance in modern society.
This deep dive into the electron configuration of cobalt underscores the intimate relationship between atomic structure and macroscopic properties. By understanding the fundamental principles of electronic structure, we can better appreciate the remarkable diversity and utility of chemical elements like cobalt.
Latest Posts
Latest Posts
-
Quantitative Analysis Of Vinegar Via Titration
Mar 29, 2025
-
Definition Of Order Of A Reaction
Mar 29, 2025
-
Why Is Blood Clotting A Positive Feedback
Mar 29, 2025
-
What Elements Are Most To Become Anions
Mar 29, 2025
-
Which Place On An Enzyme Binds A Substrate
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration For Cobalt . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
