Melting Point Trends In The Periodic Table
Muz Play
Mar 23, 2025 · 7 min read
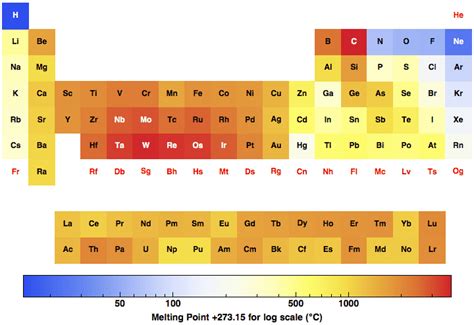
Table of Contents
Melting Point Trends in the Periodic Table: A Comprehensive Guide
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. One such property, crucial for understanding material behavior, is the melting point. This article delves deep into the fascinating trends observed in melting points across the periodic table, explaining the underlying forces and exceptions that make this topic so rich and complex. We'll explore the impact of atomic size, bonding type, and other factors that govern how readily an element transitions from a solid to a liquid state.
Understanding Melting Point
The melting point of a substance is the temperature at which it changes from a solid to a liquid state at atmospheric pressure. This transition occurs when the thermal energy overcomes the intermolecular forces holding the atoms or molecules in a fixed crystalline structure. Stronger intermolecular forces require more energy (higher temperature) to overcome, resulting in a higher melting point.
Periodic Trends: A General Overview
While no single, perfectly predictable trend governs melting points across the entire periodic table, some general patterns emerge based on the arrangement of elements and their electronic configurations. These trends are influenced primarily by:
-
Atomic Size: Generally, larger atoms have weaker interatomic forces, leading to lower melting points. As you move down a group, atomic size increases, and melting points often decrease (with exceptions, as we'll see later).
-
Atomic Mass: Heavier atoms, with greater mass, often exhibit stronger London dispersion forces (a type of van der Waals force), which can contribute to a higher melting point. However, this effect is less significant than the impact of atomic size and bonding type.
-
Bonding Type: The type of chemical bond—metallic, covalent, or ionic—dramatically affects the melting point. Metallic bonds, with delocalized electrons, tend to result in relatively high melting points, especially for transition metals. Covalent network solids, like diamond, have extremely high melting points due to the strong covalent bonds throughout the structure. Ionic compounds exhibit a wide range of melting points depending on the charge and size of the ions involved, but generally have relatively high melting points compared to molecular substances.
Across Periods (Rows): Exploring the Horizontal Trends
Let's examine the melting point trends across periods (rows) of the periodic table.
Period 2 (Li to Ne): A Diverse Landscape
Period 2 showcases a significant range of melting points. Lithium (Li), a soft alkali metal, has a relatively low melting point due to its weak metallic bonding. As we move across, the melting points generally increase, reaching a peak with carbon (C) in its diamond allotrope. Diamond's incredibly high melting point reflects the strength of its covalent network bonds. Subsequently, the melting points of nitrogen (N) and oxygen (O) are significantly lower, existing as diatomic molecules with weak intermolecular forces. Finally, neon (Ne), a noble gas, possesses an extremely low melting point because of its weak London dispersion forces.
Period 3 (Na to Ar): Similar Patterns, Subtle Differences
Similar trends are observed in period 3 (Na to Ar). Sodium (Na), like lithium, exhibits a low melting point. Silicon (Si) displays a higher melting point than aluminum (Al) due to stronger covalent bonds in its network structure. Phosphorus (P), sulfur (S), chlorine (Cl), and argon (Ar) exhibit successively lower melting points, reflecting the decreasing strength of intermolecular forces as we approach the noble gases.
Later Periods: Increasing Complexity
The trends in later periods become more complex due to the increasing number of electrons and the variety of bonding possibilities. While the general pattern of initially increasing melting points followed by a decrease remains, the influence of d and f orbitals introduces more intricate interactions that affect the strength of metallic bonding, leading to exceptions and irregularities.
Down Groups (Columns): The Vertical Trends
Moving down a group (column) in the periodic table, atomic size generally increases, and the melting points often show a decrease. However, this trend is not without exceptions, particularly for the transition metals and some other groups.
Alkali Metals (Group 1): Decreasing Melting Points
The alkali metals, from lithium (Li) to francium (Fr), demonstrate a clear decreasing trend in melting points. While the increasing atomic size contributes to this, the relatively weak metallic bonding remains a crucial factor.
Alkaline Earth Metals (Group 2): A More Complex Pattern
The alkaline earth metals (Be to Ra) also show a general decrease in melting points down the group, but the trend is less consistent than in group 1. Beryllium (Be) and Magnesium (Mg) have unusually high melting points compared to their larger counterparts, attributed to stronger metallic bonding.
Halogens (Group 17): Increasing Melting Points (with a twist)
The halogens (F to At) exhibit an increasing trend in melting points as we move down the group. This is somewhat unexpected given the increase in atomic size. The explanation lies in the increasing strength of the London dispersion forces with increasing molecular size and mass. Fluorine and Chlorine exist as diatomic molecules with relatively weak intermolecular forces. Bromine is a liquid, and iodine is a solid, at room temperature, reflecting the gradual increase in intermolecular forces.
Noble Gases (Group 18): Always Low, but a Gradual Increase
Noble gases are known for their extremely low melting points due to the weak London dispersion forces. However, even amongst noble gases, there's a subtle upward trend in melting points as you progress down the group, reflecting the stronger dispersion forces associated with increasing atomic mass and size.
Exceptions and Irregularities: Why the Simple Rules Don't Always Apply
While the general trends outlined above provide a valuable framework, numerous exceptions exist. These exceptions highlight the complex interplay of factors that determine melting points:
-
Allotropes: Elements can exist in different allotropic forms, each with unique structures and melting points. Carbon, for instance, has diamond (very high melting point) and graphite (moderately high melting point) allotropes.
-
Crystal Structure: The arrangement of atoms in a solid's crystal lattice significantly influences its melting point. Different crystal structures lead to varying interatomic interactions and, consequently, different melting points.
-
Electron Configuration: The electron configuration of an atom impacts its bonding behavior and, thus, its melting point. Transition metals, with their partially filled d orbitals, often display unique melting point patterns due to intricate d-electron interactions.
-
Intermolecular Forces: Beyond the main bonding types, various weaker intermolecular forces such as hydrogen bonding, dipole-dipole interactions, and London dispersion forces play significant roles, particularly in molecular compounds. These forces can either enhance or weaken the overall interatomic/intermolecular interactions, leading to deviations from the general trends.
Applications and Significance
Understanding melting point trends is crucial in diverse fields:
-
Material Science: Predicting and manipulating melting points is vital in designing new materials with specific properties. This knowledge is used to create alloys with desired melting temperatures for various applications.
-
Geochemistry: Melting points provide insights into geological processes. For example, knowing the melting points of minerals helps geologists understand rock formation and volcanic activity.
-
Chemical Engineering: Controlling the melting points of substances is vital in numerous chemical processes, including separation techniques, crystal growth, and material processing.
-
Chemistry: The melting point is a critical physical property used to identify and characterize substances.
Conclusion
Melting point trends in the periodic table offer a fascinating glimpse into the intricate relationship between atomic structure, bonding, and physical properties. While general trends exist, numerous exceptions highlight the complexity of interatomic and intermolecular interactions. Understanding these trends and their underlying mechanisms is crucial for advancing knowledge across various scientific and engineering disciplines. Further research continues to refine our understanding of the factors that govern melting points and expand our ability to predict and control these important material properties. By appreciating the interplay of atomic size, bonding type, crystal structure, and intermolecular forces, we can gain a deeper understanding of the periodic table's rich tapestry of physical properties and their significance in the world around us.
Latest Posts
Latest Posts
-
Divides The Body Into Superior And Inferior Sections
Mar 25, 2025
-
Learn Spanish Book Pdf Free Download
Mar 25, 2025
-
Has A Definite Volume And Shape
Mar 25, 2025
-
Group 5a On The Periodic Table
Mar 25, 2025
-
A Neutron Has Approximately The Same Mass As
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Melting Point Trends In The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
