Metals Account For Two-thirds Of Elements In The Periodic Table
Muz Play
Mar 29, 2025 · 7 min read
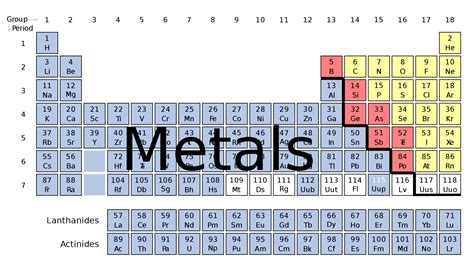
Table of Contents
Metals: The Backbone of the Periodic Table
Metals constitute a significant portion of the elements found on the periodic table, accounting for approximately two-thirds of all known elements. Their prevalence and diverse properties make them fundamental to our understanding of chemistry, physics, and the world around us. This article will delve into the fascinating world of metals, exploring their characteristics, classification, applications, and their crucial role in shaping modern society.
Defining Metals: A Look at Their Properties
Before delving into the specifics, it's crucial to establish a clear understanding of what defines a metal. While there's no single, universally accepted definition, several key characteristics collectively distinguish metals from non-metals:
1. Physical Properties:
- Conductivity: Metals are renowned for their excellent electrical and thermal conductivity. This property stems from the unique structure of their atoms, allowing electrons to move freely throughout the metallic lattice. This ease of electron movement explains why metals are used extensively in electrical wiring and heat exchangers.
- Malleability and Ductility: Metals are generally malleable (capable of being hammered into thin sheets) and ductile (capable of being drawn into wires). This ability to deform under stress without fracturing is a direct consequence of the ability of metal atoms to slide past one another within the crystal structure.
- Luster: Most metals exhibit a characteristic metallic luster, reflecting light efficiently. This property contributes to their widespread use in decorative applications.
- Density: Metals tend to have high densities compared to non-metals, meaning they possess a large amount of mass packed into a small volume. However, there are exceptions to this rule, with some metals being relatively lightweight.
- Hardness: While many metals are hard, this isn't a universally applicable characteristic. The hardness of a metal can vary considerably depending on its atomic structure and the presence of impurities or alloying elements.
2. Chemical Properties:
- Electron Configuration: Metals typically have few electrons in their outermost shell (valence electrons). This configuration makes them prone to losing electrons to achieve a stable electron configuration, a characteristic that defines their reactivity.
- Ionization Energy: Metals have relatively low ionization energies, indicating it takes relatively little energy to remove an electron from a metal atom. This contributes to their ability to form positive ions (cations) in chemical reactions.
- Electropositivity: Metals are electropositive, meaning they readily lose electrons to become positively charged ions. This characteristic is central to their participation in redox reactions (reduction-oxidation reactions involving electron transfer).
Classification of Metals: A Diverse Family
The periodic table organizes elements based on their properties, providing a framework for classifying metals. While there's some overlap, metals can be broadly categorized into:
1. Alkali Metals (Group 1):
These highly reactive metals are characterized by their single valence electron, making them eager to participate in chemical reactions. Their low melting points and softness distinguish them from other metals. Examples include lithium (Li), sodium (Na), and potassium (K).
2. Alkaline Earth Metals (Group 2):
With two valence electrons, these metals are less reactive than alkali metals but still participate readily in chemical reactions. They are generally harder and have higher melting points than alkali metals. Examples include beryllium (Be), magnesium (Mg), and calcium (Ca).
3. Transition Metals (Groups 3-12):
This large group forms the heart of the periodic table. Transition metals exhibit variable oxidation states, meaning they can lose different numbers of electrons in chemical reactions. This leads to the formation of numerous compounds with diverse properties. Examples include iron (Fe), copper (Cu), and gold (Au). Many transition metals are crucial for biological processes and industrial applications.
4. Post-Transition Metals:
Located between the transition metals and non-metals, these metals exhibit properties intermediate between the two groups. They are less reactive than alkali and alkaline earth metals and tend to have lower melting and boiling points than transition metals. Examples include aluminum (Al), tin (Sn), and lead (Pb).
5. Lanthanides and Actinides:
These two series of elements, located at the bottom of the periodic table, are often referred to as inner transition metals. They are chemically similar within their respective series and exhibit unique magnetic and optical properties. Lanthanides are relatively abundant in the earth's crust, while actinides are largely synthetically produced radioactive elements.
6. Metalloids (Semi-metals):
Located along the border between metals and non-metals, metalloids possess properties of both groups. Their behavior can vary depending on the specific conditions. Examples include silicon (Si) and germanium (Ge).
Applications of Metals: Shaping Modern Society
The remarkable diversity of metal properties makes them indispensable in almost every aspect of modern life. Their applications span various sectors, including:
1. Construction and Infrastructure:
Steel, iron alloys, and aluminum are extensively used in construction, providing structural support for buildings, bridges, and infrastructure projects. Their strength, durability, and relative affordability make them ideal for these applications.
2. Transportation:
Metals are fundamental components of automobiles, aircraft, ships, and trains. Aluminum alloys are used in lightweight vehicles, while steel and other high-strength alloys are employed in structural components.
3. Electronics and Technology:
Copper and other conductive metals are essential components of electrical circuits, wiring, and electronic devices. Precious metals like gold and silver are used in high-tech applications due to their excellent conductivity and corrosion resistance.
4. Energy Production and Storage:
Metals play a critical role in energy production and storage technologies. Metals are components in solar cells, wind turbines, and batteries.
5. Medical Applications:
Metals are used in numerous medical applications, including implants, surgical instruments, and medical devices. Titanium alloys are favored for their biocompatibility and strength.
6. Catalysis:
Metals and metal compounds act as catalysts in numerous industrial processes, accelerating chemical reactions without being consumed themselves. This catalytic activity is crucial in the production of various chemicals and materials.
The Importance of Understanding Metals
The dominance of metals in the periodic table reflects their profound impact on our lives. From the simplest tools to the most sophisticated technologies, metals are indispensable to modern society. Further research into metal properties and their applications is essential for developing new materials, improving existing technologies, and addressing global challenges such as sustainable energy and resource management. A deeper understanding of metals’ chemical and physical properties allows us to leverage their unique capabilities in innovative and sustainable ways. The ongoing exploration of the periodic table and its metallic elements ensures continued progress and development in numerous scientific and technological fields.
Environmental Considerations and Sustainability
While metals are essential, their extraction, processing, and disposal pose environmental challenges. Mining activities can cause habitat destruction and water pollution. The manufacturing process often involves energy-intensive steps and can generate harmful emissions. Sustainable practices, including responsible mining, recycling, and the development of less environmentally damaging extraction methods, are crucial to mitigating these negative impacts. Research into alternative materials and more efficient metal recycling processes are critical to promoting a sustainable future.
Future Directions in Metal Research
Research into metals continues to expand, driven by the need for novel materials with enhanced properties. This includes:
- Advanced Alloys: The development of alloys with enhanced strength, corrosion resistance, and other desirable properties is a major area of research. This includes exploring new alloy compositions and refining existing ones.
- Nanomaterials: Nanotechnology offers the possibility of creating metal-based materials with unique properties and applications, such as enhanced catalytic activity or improved electrical conductivity.
- Bio-inspired Materials: Researchers are studying the structure and properties of biological materials to design new metal-based materials with improved functionality and biocompatibility.
- Metal Recycling and Recovery: Improved metal recycling technologies are crucial for sustainable resource management and reducing environmental impacts.
The two-thirds of the periodic table occupied by metals highlight their significance in science, technology, and daily life. Continued research and innovation in this field will be instrumental in shaping a sustainable and technologically advanced future. Understanding the intricate relationships between the structure, properties, and applications of metals is crucial for progress in diverse fields and for creating a more sustainable and resilient world.
Latest Posts
Latest Posts
-
What Is The Coefficient Of Restitution
Mar 31, 2025
-
What Are The Basic Units Of All Living Things
Mar 31, 2025
-
The Spoils System Allocated Political Appointments On The Basis Of
Mar 31, 2025
-
What Is A Conjugated Pi System
Mar 31, 2025
-
What Does Mic Stand For In Microbiology
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Metals Account For Two-thirds Of Elements In The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
