Mg Oh 2 Is Acid Or Base
Muz Play
Mar 29, 2025 · 4 min read
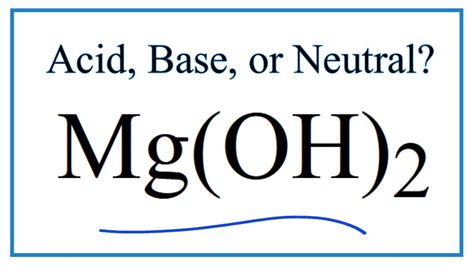
Table of Contents
Mg(OH)₂: Acid or Base? Understanding Magnesium Hydroxide's Properties
Magnesium hydroxide, Mg(OH)₂, is a chemical compound that sparks curiosity, particularly when classifying it as an acid or a base. This comprehensive article delves deep into the chemical properties of Mg(OH)₂, exploring its behavior in aqueous solutions, its practical applications, and its significance in various fields. We will definitively answer the question: Is Mg(OH)₂ an acid or a base? and explore the nuances of its behavior.
Understanding Acids and Bases
Before classifying Mg(OH)₂, it's crucial to understand the fundamental definitions of acids and bases. Several theories define these concepts, the most relevant for this discussion being the Arrhenius and Brønsted-Lowry theories.
Arrhenius Theory
The Arrhenius theory, proposed by Svante Arrhenius, defines acids as substances that produce hydrogen ions (H⁺) when dissolved in water, and bases as substances that produce hydroxide ions (OH⁻) when dissolved in water.
Brønsted-Lowry Theory
The Brønsted-Lowry theory, a broader definition, defines acids as proton (H⁺) donors and bases as proton acceptors. This theory extends the definition beyond aqueous solutions.
Classifying Mg(OH)₂: A Definitive Answer
According to both Arrhenius and Brønsted-Lowry theories, Mg(OH)₂ is a base. When dissolved in water, magnesium hydroxide dissociates into magnesium ions (Mg²⁺) and hydroxide ions (OH⁻):
Mg(OH)₂(s) → Mg²⁺(aq) + 2OH⁻(aq)
The presence of hydroxide ions (OH⁻) in the solution is the key indicator of its basic nature. These hydroxide ions increase the concentration of OH⁻ in the solution, leading to an increase in pH (a measure of acidity or alkalinity). A pH greater than 7 indicates a basic solution.
The pH of Mg(OH)₂ Solutions
The pH of a magnesium hydroxide solution depends on its concentration. Highly concentrated solutions will exhibit a significantly higher pH than dilute solutions. However, even dilute solutions will still show a pH above 7, confirming its basic nature. The relatively low solubility of Mg(OH)₂ in water limits the concentration of OH⁻ ions, resulting in a moderately alkaline solution rather than a highly caustic one.
Practical Applications of Mg(OH)₂'s Basic Properties
The basic properties of Mg(OH)₂ are exploited in various applications:
1. Antacids and Laxatives: Neutralizing Stomach Acid
Mg(OH)₂ is a common ingredient in antacids due to its ability to neutralize stomach acid (hydrochloric acid, HCl). The reaction between Mg(OH)₂ and HCl is:
Mg(OH)₂(s) + 2HCl(aq) → MgCl₂(aq) + 2H₂O(l)
This reaction neutralizes the excess acid, relieving heartburn and indigestion. Mg(OH)₂ also acts as a mild laxative, drawing water into the intestines and stimulating bowel movements.
2. Fire Retardant: Preventing Combustion
Magnesium hydroxide is used as a fire retardant in various materials. When heated, Mg(OH)₂ undergoes dehydration, releasing water vapor:
Mg(OH)₂(s) → MgO(s) + H₂O(g)
This endothermic reaction absorbs a significant amount of heat, reducing the temperature of the surrounding material and slowing down the combustion process. The released water vapor further helps to extinguish flames.
3. Water Treatment: Removing Impurities
Mg(OH)₂ is used in water treatment to remove impurities, particularly phosphates and other contaminants. It acts as a coagulant, helping to settle out suspended solids.
4. Production of Magnesium Compounds
Mg(OH)₂ serves as a crucial precursor in the synthesis of various magnesium compounds. Its reaction with acids forms different magnesium salts, which are vital in various industries.
Comparing Mg(OH)₂ with Other Compounds
Understanding the basic nature of Mg(OH)₂ becomes clearer when comparing it with other compounds.
MgO: Magnesium Oxide
Magnesium oxide (MgO) is also a basic compound. However, it is a stronger base than Mg(OH)₂ because it reacts more readily with acids. MgO does not directly produce hydroxide ions in water, instead reacting with water to form Mg(OH)₂.
HCl: Hydrochloric Acid
Hydrochloric acid (HCl) is a strong acid, the opposite of Mg(OH)₂. It readily donates protons (H⁺) in aqueous solutions, significantly lowering the pH.
NaOH: Sodium Hydroxide
Sodium hydroxide (NaOH) is a strong base, similar to Mg(OH)₂ in its basic properties. However, NaOH is far more soluble in water, leading to a more highly alkaline solution compared to Mg(OH)₂.
Safety Precautions when Handling Mg(OH)₂
While Mg(OH)₂ is generally considered safe at low concentrations, certain precautions should be taken:
- Eye contact: Avoid direct contact with eyes. Rinse thoroughly with water if contact occurs.
- Inhalation: Avoid inhalation of dust. Use appropriate respiratory protection if necessary.
- Ingestion: While used in antacids, large quantities can have laxative effects. Consult a doctor if ingested.
- Disposal: Dispose of Mg(OH)₂ waste according to local regulations.
Conclusion: Mg(OH)₂ - A Versatile Basic Compound
In conclusion, Mg(OH)₂ is unequivocally a base. Its ability to produce hydroxide ions in water, its reaction with acids, and its various applications all strongly support this classification. Understanding its basic nature is essential for its safe and effective use in diverse fields, from medicine and fire safety to water treatment and industrial chemical processes. The unique properties of magnesium hydroxide, coupled with its relatively low toxicity, make it a valuable and versatile compound with a wide range of applications. Further research continues to explore new and innovative uses for this important chemical.
Latest Posts
Latest Posts
-
The Variance Is The Square Root Of The Standard Deviation
Apr 01, 2025
-
Easy Way To Find Common Multiples
Apr 01, 2025
-
What Is The Structural And Functional Unit Of The Kidney
Apr 01, 2025
-
Evolutionary Relationships Between Organisms Are Determined By
Apr 01, 2025
-
Do Polar Covalent Bonds Share Electrons Equally
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Mg Oh 2 Is Acid Or Base . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
