Predict The Charge That A Calcium Ion Would Have
Muz Play
Mar 15, 2025 · 5 min read
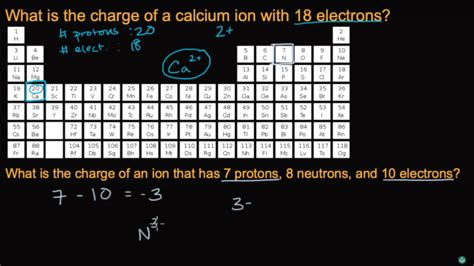
Table of Contents
Predicting the Charge of a Calcium Ion: A Deep Dive into Atomic Structure and Ionic Bonding
Predicting the charge of a calcium ion is a fundamental concept in chemistry, stemming from an understanding of atomic structure and the principles of ionic bonding. This article will delve into the intricacies of this prediction, exploring the electron configuration of calcium, its reactivity, and the resulting ionic charge. We'll also discuss the broader implications of ionic charge in various chemical contexts.
Understanding Atomic Structure: The Key to Predicting Ionic Charge
The key to predicting the charge of any ion lies in understanding its atomic structure, specifically the arrangement of electrons within its electron shells. Atoms strive for stability, often achieved by achieving a full outer electron shell (also known as the valence shell). This stable configuration is often associated with the noble gases, which have eight electrons in their outer shell (except for helium, which has two).
Calcium's Electron Configuration: The Foundation of its Reactivity
Calcium (Ca) has an atomic number of 20, meaning it possesses 20 protons and 20 electrons in a neutral atom. Its electron configuration is written as 1s²2s²2p⁶3s²3p⁶4s². This configuration shows the distribution of electrons across different energy levels (shells) and sublevels (orbitals). The outermost shell (the 4s shell) contains two electrons.
The Octet Rule and the Drive for Stability
The octet rule states that atoms tend to gain, lose, or share electrons in order to achieve a full outer electron shell of eight electrons. This configuration provides maximum stability. Calcium, with its two valence electrons, is more stable by losing these two electrons rather than gaining six to achieve a full octet. This is because losing two electrons requires significantly less energy than gaining six.
Calcium's Ionization and the Formation of Ca²⁺
The process of losing electrons from an atom is called ionization. When calcium loses its two valence electrons, it forms a cation – a positively charged ion. Since each electron carries a negative charge (-1), losing two electrons results in a net positive charge of +2. This is why the calcium ion is denoted as Ca²⁺.
Ionization Energy and the Ease of Electron Loss
The energy required to remove an electron from an atom is called ionization energy. The first ionization energy (removing the first electron) is relatively low for calcium, indicating that it is relatively easy to remove the first electron. The second ionization energy (removing the second electron) is also relatively low, further reinforcing the tendency of calcium to lose two electrons. Subsequent ionization energies are substantially higher.
The Role of Electronegativity
Electronegativity is a measure of an atom's ability to attract electrons towards itself in a chemical bond. Calcium has a relatively low electronegativity. This means it doesn't strongly attract electrons, making it more likely to lose electrons rather than gain them. This low electronegativity contributes to its tendency to form a positive ion.
Ionic Bonding: The Interaction between Ions
The Ca²⁺ ion doesn't exist in isolation. It participates in ionic bonds with other atoms, usually those with high electronegativity, such as halogens (e.g., chlorine, fluorine) or oxygen. In ionic bonding, the electrostatic attraction between the positively charged cation (Ca²⁺) and the negatively charged anion (e.g., Cl⁻, O²⁻) forms a stable ionic compound.
Examples of Calcium Compounds
- Calcium Chloride (CaCl₂): Each calcium ion (Ca²⁺) bonds with two chloride ions (Cl⁻) to form a neutral compound. The positive and negative charges balance each other out.
- Calcium Oxide (CaO): One calcium ion (Ca²⁺) bonds with one oxide ion (O²⁻) to form a neutral compound. Again, the charges balance each other.
- Calcium Sulfate (CaSO₄): One calcium ion (Ca²⁺) bonds with one sulfate ion (SO₄²⁻) to form a neutral compound. The charges balance.
Beyond the Basics: Exploring More Complex Scenarios
While predicting the charge of a calcium ion is straightforward in simple ionic compounds, more complex scenarios exist. These complexities arise in coordination compounds, organometallic chemistry, and other advanced chemical systems.
Coordination Compounds and Variable Oxidation States (though not relevant for Calcium)
In coordination compounds, transition metals can often exhibit variable oxidation states, meaning they can form ions with different charges depending on the ligands bound to them. However, calcium, as an alkaline earth metal, typically maintains a +2 oxidation state. Its electronic configuration and the significant energy required to remove a third electron prevent it from forming ions with higher charges.
Influence of Lattice Energy in Crystal Structures
The stability of ionic compounds is also influenced by lattice energy, which is the energy released when ions come together to form a crystal lattice. The strong electrostatic attraction between Ca²⁺ and anions contributes significantly to the high lattice energy of many calcium-containing compounds.
Practical Applications and Significance of Calcium Ions
Calcium ions play crucial roles in various biological and industrial processes:
Biological Significance of Calcium Ions
- Bone structure: Calcium ions are essential components of bones and teeth, providing structural strength and support.
- Muscle contraction: Calcium ions are crucial for muscle contraction and relaxation.
- Nerve impulse transmission: Calcium ions are involved in nerve impulse transmission and synaptic signaling.
- Blood clotting: Calcium ions are vital for blood clotting processes.
Industrial Applications of Calcium Compounds
Calcium compounds have numerous industrial applications:
- Cement production: Calcium compounds are key components in the production of cement, a fundamental material in construction.
- Plaster and gypsum: Calcium sulfate (gypsum) is used in the production of plaster and drywall.
- Fertilizers: Calcium compounds are essential nutrients in fertilizers, promoting healthy plant growth.
- Food additive: Calcium compounds are used as food additives to enhance nutritional value and texture.
Conclusion: Predicting the Charge of a Calcium Ion
Predicting the charge of a calcium ion, as Ca²⁺, is a relatively straightforward application of basic chemical principles. Understanding its electron configuration, the octet rule, ionization energies, and electronegativity allows for a clear prediction. However, understanding the context of ionic bonding and its impact on the properties of calcium compounds and its various biological and industrial roles provide a more complete understanding of the significance of this simple prediction. The +2 charge is a cornerstone of calcium's reactivity and its widespread importance in the natural world and in various industrial processes. This fundamental understanding serves as a building block for further exploration in more advanced chemistry.
Latest Posts
Latest Posts
-
Factors That Influence The Elasticity Of Supply
Mar 17, 2025
-
Describe How The Atoms In A Compound Are Held Together
Mar 17, 2025
-
How Is Absorbance Linked To Rate Of Reaction
Mar 17, 2025
-
What Happens To Electrons In An Ionic Bond
Mar 17, 2025
-
Are Antiparalell Beta Sheets Mrore Stable
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Predict The Charge That A Calcium Ion Would Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
