Recognizing Dipole Dipole Vs London In Lewis Structures
Muz Play
Mar 17, 2025 · 7 min read
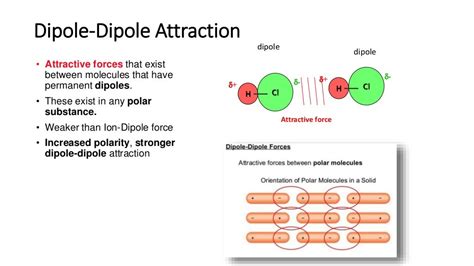
Table of Contents
Recognizing Dipole-Dipole vs. London Dispersion Forces in Lewis Structures
Understanding intermolecular forces is crucial for predicting the physical properties of molecules, such as boiling point, melting point, and solubility. Two common types of intermolecular forces are dipole-dipole interactions and London dispersion forces (LDFs). While both affect the behavior of molecules, they differ significantly in their strength and origin. This article will delve into the intricacies of distinguishing between dipole-dipole forces and London dispersion forces, using Lewis structures as a primary tool for analysis.
Understanding Intermolecular Forces
Before we dive into the specifics of differentiating dipole-dipole and London dispersion forces, let's establish a clear understanding of intermolecular forces in general. These are the attractive forces that exist between molecules, as opposed to intramolecular forces (bonds within a molecule). Intermolecular forces are generally weaker than intramolecular forces, but they significantly influence the macroscopic properties of substances.
The strength of intermolecular forces dictates the state of matter at a given temperature and pressure. Stronger intermolecular forces lead to higher boiling points, melting points, and greater viscosity.
Dipole-Dipole Forces: The Dance of Polar Molecules
Dipole-dipole forces occur between polar molecules. A polar molecule is one that possesses a permanent dipole moment, meaning it has a slightly positive end and a slightly negative end due to an uneven distribution of electron density. This uneven distribution arises from differences in electronegativity between atoms within the molecule. Electronegativity is the ability of an atom to attract electrons in a chemical bond.
How to identify a polar molecule using Lewis structures:
- Draw the Lewis structure: This shows the arrangement of atoms and bonding electrons.
- Determine the molecular geometry: Using VSEPR theory (Valence Shell Electron Pair Repulsion), predict the three-dimensional shape of the molecule. This is crucial because the geometry determines the symmetry of the charge distribution.
- Check for polar bonds: Look for bonds between atoms with significantly different electronegativities. The greater the electronegativity difference, the more polar the bond. A common electronegativity scale is the Pauling scale.
- Assess molecular symmetry: If the molecule is symmetrical (e.g., linear CO₂, tetrahedral CH₄), the individual bond dipoles cancel each other out, resulting in a nonpolar molecule despite having polar bonds. However, if the molecule is asymmetrical (e.g., bent H₂O, trigonal pyramidal NH₃), the bond dipoles do not cancel, leading to a net dipole moment and a polar molecule.
Example: Consider water (H₂O). The Lewis structure shows two O-H bonds. Oxygen is significantly more electronegative than hydrogen, creating polar bonds. The bent molecular geometry prevents the bond dipoles from canceling, resulting in a net dipole moment, making water a polar molecule. Therefore, water molecules exhibit dipole-dipole interactions.
London Dispersion Forces: The Fleeting Attractions
London Dispersion Forces (LDFs), also known as instantaneous dipole-induced dipole forces, are the weakest type of intermolecular force. They arise from temporary, instantaneous fluctuations in electron distribution around atoms and molecules. Even in nonpolar molecules, where electrons are, on average, evenly distributed, there are momentary imbalances. At any given instant, the electron cloud might be slightly more concentrated on one side of the molecule than the other, creating a temporary, instantaneous dipole. This temporary dipole can induce a dipole in a neighboring molecule, leading to a weak attractive force.
LDFs are present in all molecules, regardless of their polarity. However, their strength increases with the size and shape of the molecule. Larger molecules have more electrons, increasing the likelihood of instantaneous dipole formation and stronger LDFs. A more elongated shape also increases the surface area available for interaction, further enhancing LDFs.
Identifying LDFs from Lewis structures: While you can't directly "see" LDFs in a Lewis structure, their presence is implied by the existence of any molecule. The larger the molecule (as indicated by the number of atoms and electrons in the Lewis structure), the stronger the LDFs.
Example: Consider methane (CH₄). The Lewis structure shows a tetrahedral molecule with symmetrical distribution of electrons. While the individual C-H bonds are slightly polar, the symmetry leads to a nonpolar molecule. Therefore, the predominant intermolecular force in methane is London dispersion forces. However, because methane is a relatively small molecule, the LDFs are relatively weak.
Distinguishing Dipole-Dipole from London Dispersion Forces: A Practical Guide
The key difference lies in the permanence of the dipole moment. Dipole-dipole forces involve permanent dipoles arising from the inherent polarity of the molecule, while LDFs involve temporary, instantaneous dipoles.
Here's a step-by-step guide to differentiate between the two using Lewis structures:
- Draw the Lewis structure: This provides the foundation for all subsequent analyses.
- Determine molecular geometry: Use VSEPR theory to predict the three-dimensional shape.
- Identify polar bonds: Look for bonds between atoms with different electronegativities.
- Assess molecular symmetry: If the molecule is symmetrical, the bond dipoles cancel, and the molecule is nonpolar, meaning the dominant intermolecular force is LDFs.
- Presence of a net dipole moment: If the molecule is asymmetrical and possesses a net dipole moment (meaning the bond dipoles don't cancel), the molecule is polar, and dipole-dipole forces are significant. However, remember that all molecules experience LDFs, even those with strong dipole-dipole interactions. LDFs simply contribute to a lesser extent.
- Consider molecular size: For molecules of similar size and polarity, the strength of LDFs will be comparable. However, for molecules of significantly different sizes, even if both have dipole-dipole interactions, the larger molecule will likely have stronger overall intermolecular forces due to stronger LDFs.
Examples to Clarify the Distinction
Let's analyze several examples to solidify our understanding:
1. Carbon Dioxide (CO₂):
- Lewis structure: O=C=O
- Molecular geometry: Linear
- Polar bonds: Yes (C=O bonds are polar)
- Molecular symmetry: Symmetrical; the bond dipoles cancel.
- Intermolecular forces: Primarily London dispersion forces.
2. Water (H₂O):
- Lewis structure: H-O-H (with lone pairs on O)
- Molecular geometry: Bent
- Polar bonds: Yes (O-H bonds are polar)
- Molecular symmetry: Asymmetrical; bond dipoles do not cancel, resulting in a net dipole moment.
- Intermolecular forces: Dipole-dipole forces are dominant, with LDFs also present.
3. Methane (CH₄):
- Lewis structure: Tetrahedral structure with C in the center and four H atoms.
- Molecular geometry: Tetrahedral
- Polar bonds: Slightly polar C-H bonds.
- Molecular symmetry: Symmetrical; bond dipoles cancel.
- Intermolecular forces: Primarily London dispersion forces.
4. Chloromethane (CH₃Cl):
- Lewis structure: Tetrahedral structure with C in the center, three H atoms, and one Cl atom.
- Molecular geometry: Tetrahedral
- Polar bonds: C-Cl bond is significantly polar.
- Molecular symmetry: Asymmetrical; the C-Cl bond dipole doesn't cancel.
- Intermolecular forces: Dipole-dipole forces are present due to the polar C-Cl bond. LDFs also contribute, but to a lesser extent compared to a molecule of similar size without a dipole.
5. Bromomethane (CH₃Br):
- Lewis structure: Similar to chloromethane, but with Br instead of Cl.
- Molecular geometry: Tetrahedral
- Polar bonds: C-Br bond is polar (though less polar than C-Cl).
- Molecular symmetry: Asymmetrical, with a net dipole moment.
- Intermolecular forces: Dipole-dipole forces and LDFs. Since Bromine is larger than Chlorine, the London Dispersion Forces will be stronger than in chloromethane.
Conclusion
Differentiating between dipole-dipole forces and London dispersion forces is essential for understanding the behavior of molecules. By systematically analyzing Lewis structures and considering molecular geometry and symmetry, we can effectively determine the dominant intermolecular forces at play. Remember that while dipole-dipole forces are significant in polar molecules, all molecules experience London dispersion forces, and their contribution to the overall intermolecular interactions should not be overlooked, especially in larger molecules. This understanding allows us to predict and explain various macroscopic properties of substances, ultimately enhancing our comprehension of the world around us at a molecular level.
Latest Posts
Latest Posts
-
Do Valence Electrons Have The Most Energy
Mar 17, 2025
-
Element Vs Compound Vs Homogeneous Vs Heterogeneous
Mar 17, 2025
-
For An Exothermic Reaction The Products
Mar 17, 2025
-
Fallacies Divide Into Roughly Two Kinds
Mar 17, 2025
-
An Organism That Cannot Grow Without Oxygen Is A An
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Recognizing Dipole Dipole Vs London In Lewis Structures . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
