Reverse Phase Vs Normal Phase Hplc
Muz Play
Mar 24, 2025 · 6 min read
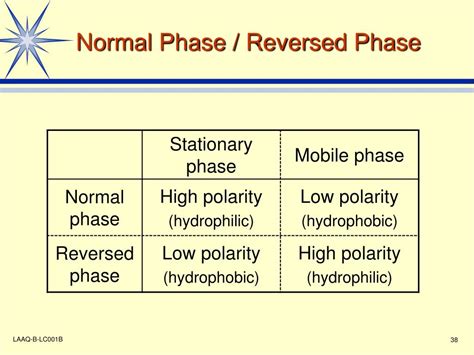
Table of Contents
Reverse Phase vs. Normal Phase HPLC: A Comprehensive Comparison
High-Performance Liquid Chromatography (HPLC) is a powerful analytical technique widely used for separating, identifying, and quantifying components in a mixture. Two primary modes dominate HPLC applications: reverse phase and normal phase. Understanding the differences between these modes is crucial for selecting the appropriate method for a given analytical task. This article provides a comprehensive comparison of reverse phase and normal phase HPLC, exploring their principles, advantages, disadvantages, and typical applications.
Understanding the Principles: Stationary and Mobile Phases
Both reverse phase and normal phase HPLC rely on the interaction between the analyte (the substance being analyzed) and the stationary and mobile phases. The stationary phase is a solid material packed within the HPLC column, while the mobile phase is a liquid solvent that carries the analyte through the column. The separation occurs based on the differential partitioning of the analyte between these two phases.
Normal Phase HPLC
In normal phase HPLC, the stationary phase is polar (e.g., silica gel) and the mobile phase is nonpolar (e.g., hexane, heptane). The separation mechanism is based on the analyte's polarity. More polar analytes interact more strongly with the polar stationary phase and elute later, while less polar analytes elute faster.
Reverse Phase HPLC
Reverse phase HPLC is the dominant mode in HPLC applications. It uses a nonpolar stationary phase (e.g., C18, C8 bonded silica) and a polar mobile phase (e.g., water, methanol, acetonitrile). In this case, the separation principle is reversed: less polar analytes interact more strongly with the nonpolar stationary phase and elute later, whereas more polar analytes elute faster.
Key Differences: A Head-to-Head Comparison
| Feature | Reverse Phase HPLC | Normal Phase HPLC |
|---|---|---|
| Stationary Phase | Nonpolar (e.g., C18, C8 bonded silica) | Polar (e.g., silica gel) |
| Mobile Phase | Polar (e.g., water, methanol, acetonitrile) | Nonpolar (e.g., hexane, heptane) |
| Separation Mechanism | Based on hydrophobicity/lipophilicity | Based on polarity |
| Analyte Interaction | Nonpolar analytes retain longer | Polar analytes retain longer |
| Solvent Strength | Increased solvent strength with increased polarity | Increased solvent strength with increased polarity |
| Column Lifetime | Generally longer | Shorter, susceptible to moisture and degradation |
| Peak Shape | Often sharper peaks | Can exhibit peak tailing |
| Gradient Elution | Easier to implement and control | More challenging to implement and control |
| Sample Cleanup | Often less critical | Often requires more extensive sample cleanup |
| Common Applications | Pharmaceuticals, environmental analysis, biomolecules | Carbohydrates, lipids, chiral separations |
Advantages and Disadvantages of Reverse Phase HPLC
Advantages:
- Versatility: Reverse phase HPLC is highly versatile and can be used to separate a wide range of compounds with varying polarities.
- Reproducibility: The method is highly reproducible, making it suitable for quantitative analysis.
- Ease of Gradient Elution: Gradient elution, where the mobile phase composition is changed during the separation, is readily implemented and controlled in reverse phase HPLC, enhancing separation efficiency.
- Longer Column Lifetime: Reverse phase columns are generally more robust and have a longer lifetime compared to normal phase columns.
- Widely Available: Reverse phase columns and solvents are readily available and relatively inexpensive.
Disadvantages:
- Peak Tailing: While often less pronounced than in normal phase, peak tailing can still occur, particularly for basic compounds.
- Secondary Interactions: Secondary interactions, such as silanol interactions, can affect peak shape and retention time, requiring careful optimization.
- Limited Applicability: Reverse phase HPLC is less suitable for highly polar compounds or those prone to strong interactions with the aqueous mobile phase.
Advantages and Disadvantages of Normal Phase HPLC
Advantages:
- Excellent for Polar Compounds: Normal phase HPLC is exceptionally well-suited for separating highly polar compounds that are difficult to analyze using reverse phase.
- High Efficiency: Under optimal conditions, normal phase HPLC can offer very high separation efficiency.
- Unique Selectivity: It provides unique selectivity that is often different from reverse phase HPLC, offering alternative separation strategies.
Disadvantages:
- Shorter Column Lifetime: Normal phase columns are more sensitive to moisture and can degrade faster than reverse phase columns.
- Reproducibility Issues: Achieving consistent results can be challenging due to the sensitivity of the stationary phase to moisture and the difficulty in controlling the mobile phase composition.
- Limited Gradient Elution: Gradient elution is more challenging to implement and control in normal phase HPLC compared to reverse phase.
- Peak Tailing: Peak tailing is a common problem in normal phase HPLC, potentially affecting quantification accuracy.
- Sample Preparation: Normal phase HPLC often requires more extensive sample preparation to remove impurities that might interfere with the separation.
Choosing Between Reverse Phase and Normal Phase HPLC
The choice between reverse phase and normal phase HPLC depends primarily on the nature of the analytes being separated.
-
Reverse phase HPLC is generally preferred for analyzing nonpolar and moderately polar compounds, especially in situations where high reproducibility, ease of gradient elution, and longer column lifetime are important. This makes it the method of choice for many pharmaceutical, environmental, and biochemical applications. Examples include the analysis of pesticides, pharmaceuticals, and lipids.
-
Normal phase HPLC is particularly useful for separating highly polar compounds such as carbohydrates, sugars, and some types of lipids, where reverse phase HPLC might not provide adequate separation. It's also favored when specific selectivity is needed that is not readily achievable using reverse phase methods. However, the challenges associated with reproducibility and column lifetime should be considered.
Optimizing HPLC Separations: Essential Considerations
Regardless of the chosen mode (reverse phase or normal phase), several factors need careful consideration to optimize HPLC separations:
- Mobile Phase Selection: The selection of the mobile phase is critical for achieving adequate separation. The mobile phase's polarity, pH, and additives (e.g., ion-pairing reagents) significantly influence the analyte's retention and peak shape.
- Column Selection: The column's dimensions (length, internal diameter), particle size, and stationary phase type heavily impact separation efficiency and resolution.
- Flow Rate: The mobile phase flow rate affects separation time and resolution. Optimization of flow rate is often necessary for achieving optimal results.
- Temperature: Temperature control can improve peak shape, reduce band broadening, and enhance separation efficiency.
- Detection Method: Choosing the appropriate detection method (e.g., UV-Vis, fluorescence, mass spectrometry) is essential for accurately detecting and quantifying the analytes of interest.
Advanced Techniques and Future Trends
The field of HPLC is constantly evolving, with new techniques and advancements continually improving separation capabilities and expanding applications. Some notable examples include:
- Ultra-High-Performance Liquid Chromatography (UHPLC): UHPLC utilizes smaller particle size columns and higher pressures, offering significantly faster separations with improved resolution compared to traditional HPLC.
- Two-Dimensional HPLC (2D-HPLC): 2D-HPLC combines two different separation modes (e.g., reverse phase and normal phase) to achieve superior separation of complex mixtures.
- Supercritical Fluid Chromatography (SFC): SFC employs supercritical fluids as the mobile phase, offering unique separation capabilities for compounds that are difficult to analyze using traditional HPLC.
Conclusion
Reverse phase and normal phase HPLC are powerful analytical techniques that offer distinct advantages and disadvantages. Selecting the appropriate mode depends on the specific analytical task and the properties of the analytes being separated. A thorough understanding of the underlying principles, advantages, disadvantages, and optimization strategies is crucial for achieving high-quality results in HPLC analysis. The ongoing development of advanced techniques further enhances the capabilities of HPLC, continuing to expand its application across diverse scientific fields.
Latest Posts
Latest Posts
-
When Can You Use Henderson Hasselbalch Equation
Mar 28, 2025
-
What Are Monomers Of Nucleic Acids
Mar 28, 2025
-
Tro Principles Of Chemistry A Molecular Approach
Mar 28, 2025
-
Coefficient Of Thermal Expansion Of Steel
Mar 28, 2025
-
Period 6 On The Periodic Table
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Reverse Phase Vs Normal Phase Hplc . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
