When Can You Use Henderson Hasselbalch Equation
Muz Play
Mar 28, 2025 · 5 min read
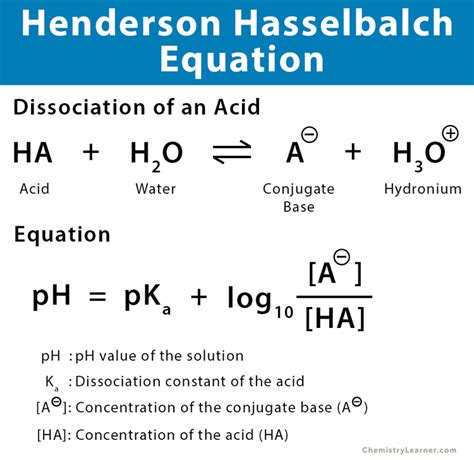
Table of Contents
When Can You Use the Henderson-Hasselbalch Equation? A Comprehensive Guide
The Henderson-Hasselbalch equation is a cornerstone of acid-base chemistry, providing a simple yet powerful tool for calculating the pH of buffer solutions. Understanding its limitations and appropriate applications is crucial for accurate chemical calculations and interpretations. This comprehensive guide delves into the scenarios where the Henderson-Hasselbalch equation shines and when it falls short, equipping you with a thorough understanding of its utility and restrictions.
Understanding the Henderson-Hasselbalch Equation
Before we explore its applications, let's refresh the equation itself:
pH = pKa + log([A⁻]/[HA])
Where:
- pH: The measure of hydrogen ion concentration (acidity) of the solution.
- pKa: The negative logarithm of the acid dissociation constant (Ka) of the weak acid. It reflects the strength of the acid; a lower pKa indicates a stronger acid.
- [A⁻]: The concentration of the conjugate base.
- [HA]: The concentration of the weak acid.
When the Henderson-Hasselbalch Equation Works Best
The Henderson-Hasselbalch equation provides accurate pH calculations under specific conditions:
1. Weak Acid-Conjugate Base Buffer Solutions: The Ideal Scenario
The equation thrives when applied to solutions containing a weak acid and its conjugate base in relatively similar concentrations. These mixtures, known as buffer solutions, resist changes in pH upon the addition of small amounts of strong acid or base. This resistance is the fundamental property that the Henderson-Hasselbalch equation helps quantify.
Example: A solution containing acetic acid (CH₃COOH) and its conjugate base, acetate (CH₃COO⁻), forms a buffer. The equation accurately predicts the pH of this buffer given the pKa of acetic acid and the concentrations of both species.
2. Calculating pH Changes upon Addition of Strong Acid or Base to a Buffer
The equation is invaluable for determining how the pH of a buffer solution will change after adding a small amount of a strong acid or base. By adjusting the concentrations of [A⁻] and [HA] to reflect the neutralization reaction, you can accurately predict the new pH. This is crucial in understanding buffer capacity and its limitations.
Example: Determining the pH change in a phosphate buffer upon addition of a small quantity of hydrochloric acid.
3. Determining the Ratio of Conjugate Base to Acid for a Desired pH
The equation can be rearranged to solve for the [A⁻]/[HA] ratio required to achieve a specific pH. This is extremely useful in preparing buffer solutions with precise pH values for various applications in chemistry, biology, and medicine.
Example: Calculating the ratio of sodium acetate to acetic acid needed to prepare a buffer solution with a pH of 4.76.
When the Henderson-Hasselbalch Equation Fails or Provides Inaccurate Results
While remarkably useful, the Henderson-Hasselbalch equation has limitations:
1. Strong Acids and Strong Bases: A Clear No-Go
The equation is not applicable to solutions of strong acids or strong bases. Strong acids and bases dissociate completely in water, violating the underlying assumption of the equation—that the weak acid only partially dissociates.
2. Concentrated Solutions: The Ionic Strength Effect
In highly concentrated solutions, the activity of ions deviates significantly from their concentration. The equation, which uses concentrations, becomes inaccurate as it fails to account for these ionic strength effects. Activity coefficients must be incorporated for more precise calculations in such scenarios.
3. Very Weak Acids or Bases (High or Low pKa Values): A Diminishing Return
For very weak acids (high pKa) or very weak bases (low pKa), the approximation inherent in the derivation of the equation breaks down. The degree of dissociation is not negligible, and more precise methods are needed for accurate pH calculations.
4. Dilute Solutions with Negligible Dissociation: Lack of Buffering Capacity
The equation assumes a significant concentration of both the weak acid and its conjugate base. If either is in very low concentration, the buffering capacity is negligible, and the pH is heavily influenced by the autoionization of water (Kw). In such cases, the equation becomes unreliable.
5. Ignoring the Autoionization of Water: Kw Considerations
The Henderson-Hasselbalch equation simplifies calculations by ignoring the contribution of water's autoionization ([H⁺][OH⁻] = Kw). This is a reasonable simplification in many buffer solutions, but it can introduce errors in highly dilute solutions or solutions with extreme pH values.
6. Temperature Dependence: Maintaining Consistency
The pKa value of a weak acid is temperature-dependent. The equation only provides accurate results if the pKa value used corresponds to the temperature of the solution. Using a pKa value from a different temperature will lead to an inaccurate pH calculation.
Beyond the Henderson-Hasselbalch Equation: More Precise Methods
For situations where the Henderson-Hasselbalch equation's limitations become significant, more rigorous methods are necessary:
- ICE tables (Initial, Change, Equilibrium): These provide a systematic approach to solving equilibrium problems, incorporating the autoionization of water and avoiding approximations.
- Activity coefficients: These correct for the deviations from ideality observed in concentrated solutions.
- Iterative numerical methods: For complex systems with multiple equilibria, these methods provide accurate solutions, although they require computational tools.
Conclusion: Knowing When and When Not to Use the Henderson-Hasselbalch Equation
The Henderson-Hasselbalch equation is an invaluable tool in acid-base chemistry, providing a quick and convenient way to estimate the pH of buffer solutions under specific conditions. However, its use requires a clear understanding of its underlying assumptions and limitations. By carefully considering the concentration, strength, and nature of the acid and base involved, you can accurately assess its applicability and choose the most suitable approach for precise pH calculations. Remember that for situations outside its ideal operating range, more sophisticated techniques are necessary for accurate results. Applying this knowledge will ensure you get reliable pH estimations and avoid misinterpretations in your chemical analyses. Remember always to consider the context and limitations when applying this valuable equation to your calculations.
Latest Posts
Latest Posts
-
What Makes Something A Strong Acid
Mar 31, 2025
-
According To The Rules Of Osmosis A System Will
Mar 31, 2025
-
Where Are Chondrocytes And Osteocytes Located
Mar 31, 2025
-
List The Types Of Persuasive Speeches
Mar 31, 2025
-
The Energy Needed To Start A Chemical Reaction Is Called
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about When Can You Use Henderson Hasselbalch Equation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
