Rows Of The Periodic Table Are Called
Muz Play
Mar 21, 2025 · 6 min read
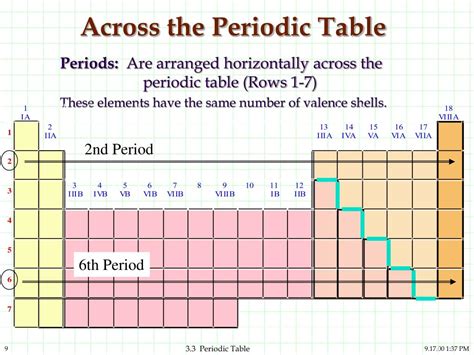
Table of Contents
Rows of the Periodic Table are Called Periods: A Deep Dive into Periodic Trends
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. Understanding its organization is crucial to grasping chemical behavior and predicting reactions. One of the fundamental aspects of this organization is the arrangement of elements into rows, formally known as periods. This article delves deep into the concept of periods, exploring their significance, the trends they reveal, and their connection to electron configuration.
What are Periods in the Periodic Table?
The horizontal rows of the periodic table are called periods. Each period represents a principal energy level or shell in an atom. As we move across a period from left to right, the number of electrons in the outermost shell (valence shell) increases. This gradual addition of electrons significantly influences the chemical properties of the elements within that period. The number of the period corresponds to the highest principal quantum number (n) of the electrons in that element's ground state electron configuration. For example, elements in Period 1 have electrons only in the n=1 shell, while elements in Period 2 have electrons in both n=1 and n=2 shells.
The Significance of Period Number
The period number provides crucial information about the electronic structure and, subsequently, the chemical behavior of an element. It directly dictates:
-
The number of electron shells: The period number indicates the number of electron shells occupied by electrons in the atom's ground state.
-
The highest energy level: The period number corresponds to the highest principal quantum number (n) of the electrons within the atom.
-
General properties: Although elements within a period exhibit varying properties, the period number gives a broad indication of their general reactivity and characteristics. For example, elements in the same period often show similar patterns in ionization energy and electronegativity trends.
-
Valence electron configuration: The period number gives clues about the possible valence electron configurations, impacting the bonding capabilities and chemical reactivity of elements within that period.
Exploring the Periods: A Detailed Look
Let's explore each period in more detail, highlighting the key characteristics and trends observed:
Period 1: The Shortest Period
Period 1 is the shortest period, containing only two elements: hydrogen (H) and helium (He). These elements possess electrons only in the n=1 shell, which can accommodate a maximum of two electrons. Hydrogen has one electron, making it highly reactive and capable of forming a single covalent bond. Helium, with a full outer shell (two electrons), is exceptionally unreactive – a noble gas.
Period 2: The Alkali Metals and Beyond
Period 2 encompasses eight elements, starting with lithium (Li), an alkali metal, and ending with neon (Ne), a noble gas. This period showcases a clear trend in properties as we progress from left to right. The alkali metals (Li, Na) are highly reactive, readily losing one electron to achieve a stable octet. Then comes the alkaline earth metals (Be, Mg), which are somewhat less reactive, losing two electrons. We then observe a progression through the transition metals (which are not fully represented in this period), before reaching the halogens (F, Cl), highly reactive non-metals that readily gain one electron. Finally, neon, with a complete octet, is a highly unreactive noble gas.
Period 3: Similar Trends, Increased Complexity
Period 3 mirrors many of the trends observed in Period 2, but with increased atomic size and slightly altered reactivity. It contains elements from sodium (Na), another alkali metal, to argon (Ar), a noble gas. The trends in electronegativity, ionization energy, and atomic radii are similar to those in Period 2, reflecting the filling of the 3s and 3p orbitals. However, the increased shielding effect of the inner electrons slightly reduces the effective nuclear charge, impacting the properties compared to their Period 2 counterparts.
Period 4 and Beyond: The Emergence of Transition Metals and Beyond
Period 4 and subsequent periods exhibit greater complexity due to the introduction of the d-block and, eventually, the f-block elements. Period 4 includes transition metals, which occupy the d-block, characterized by partially filled d orbitals. These elements demonstrate variable oxidation states and often form colored compounds. This complexity continues to increase in higher periods with the addition of the f-block elements (lanthanides and actinides).
Period Trends Across the Periodic Table
Several crucial periodic trends are observed as we move across a period:
-
Electronegativity: Electronegativity, the ability of an atom to attract electrons in a chemical bond, generally increases across a period. This is because the effective nuclear charge increases, pulling the valence electrons closer to the nucleus.
-
Ionization Energy: Ionization energy, the energy required to remove an electron from an atom, generally increases across a period. The increasing nuclear charge makes it harder to remove an electron.
-
Atomic Radius: Atomic radius, the size of an atom, generally decreases across a period. The increasing nuclear charge pulls the electrons closer to the nucleus, resulting in a smaller atomic size.
-
Metallic Character: Metallic character generally decreases across a period. Elements on the left side of a period tend to be more metallic (losing electrons readily), while those on the right are non-metallic (gaining electrons readily).
Periods and Electron Configuration
The arrangement of elements in periods is directly linked to their electron configurations. Each period represents the filling of a principal energy level. Period 1 represents the filling of the 1s subshell, Period 2 the 2s and 2p subshells, and so on. This filling pattern determines the number of valence electrons and, subsequently, the chemical behavior of the elements. Understanding electron configuration is crucial for predicting the chemical properties of an element based solely on its position in the periodic table.
The Importance of Understanding Periods
Understanding the concept of periods is fundamental to comprehending the periodic table and, by extension, chemical behavior. It allows us to:
-
Predict properties: Knowing the period number helps predict an element's general properties, such as its reactivity and electronegativity.
-
Understand bonding: The position in a period helps determine an element's bonding preferences and the types of compounds it is likely to form.
-
Analyze reactions: Understanding periodic trends enables the prediction of reaction outcomes and the identification of likely products.
-
Develop new materials: A deep understanding of periodic trends helps in the design and synthesis of new materials with specific properties.
Conclusion: Periods – A Foundation of Chemistry
Periods represent a cornerstone in the organizational structure of the periodic table. Their importance lies in their ability to reveal the fundamental trends in atomic structure and properties. By understanding the relationship between period number, electron configuration, and periodic trends, we gain a powerful tool for predicting and interpreting the behavior of chemical elements. The concept of periods, therefore, is not merely an organizational scheme but a fundamental framework for comprehending the diverse and fascinating world of chemistry. The significance of periods cannot be overstated in any serious study of chemical principles. The regular patterns observable within periods undergird a deeper understanding of the relationships between the elements and their reactions.
Latest Posts
Latest Posts
-
How Many Electrons Does Na Have
Mar 22, 2025
-
Is Freezing Water A Chemical Change
Mar 22, 2025
-
A Yellow Solid At Room Temperature And 1 Atm
Mar 22, 2025
-
Is Ots A Good Leaving Group
Mar 22, 2025
-
Normal Phase Vs Reverse Phase Hplc
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about Rows Of The Periodic Table Are Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
