Is Ots A Good Leaving Group
Muz Play
Mar 22, 2025 · 5 min read
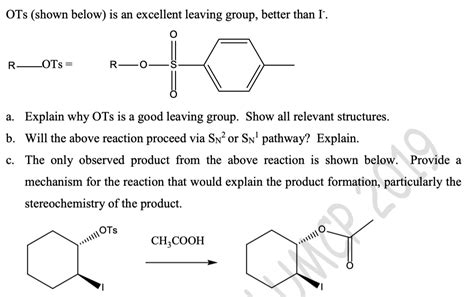
Table of Contents
Is OTS a Good Leaving Group? A Deep Dive into Leaving Group Ability
Leaving group ability is a crucial concept in organic chemistry, significantly influencing reaction rates and mechanisms. A good leaving group readily accepts electrons, stabilizing the negative charge that develops during a reaction. This article delves into the question: Is Otosylate (OTS) a good leaving group? We'll explore its properties, compare it to other common leaving groups, and examine its applications in various organic reactions.
Understanding Leaving Groups
Before assessing OTS's capabilities, let's establish the fundamentals of leaving group behavior. A good leaving group possesses several key characteristics:
-
Stability: The ability to stabilize the negative charge acquired after leaving. This often correlates with weak basicity. Strong bases are poor leaving groups because they strongly attract protons, hindering their departure.
-
Polarizability: The ability to disperse the negative charge over a larger volume. Larger, more polarizable leaving groups are generally better.
-
Resonance stabilization: The ability to delocalize the negative charge through resonance structures. This significantly enhances stability.
-
Size and steric hindrance: Bulky leaving groups can sometimes hinder reactions due to steric clashes.
Otosylate (OTS) as a Leaving Group: A Detailed Analysis
Otosylate (OTS), the tosylate ester of an alcohol, is a widely used leaving group in organic synthesis. Its structure contains a sulfonate group, which plays a significant role in its leaving group ability.
Structure and Properties:
OTS has a sulfonate group attached to an alkyl or aryl group. The sulfonate group's electron-withdrawing nature is crucial. This withdrawing effect stabilizes the negative charge that forms when the OTS group departs during a reaction. The resonance structures of the resulting tosylate anion significantly contribute to its stability.
Why OTS is a Good Leaving Group:
-
Excellent Stability: The negative charge on the departing tosylate ion is effectively delocalized across the sulfonate group through resonance. This delocalization significantly stabilizes the anion, making it a willing participant in the reaction.
-
Weak Basicity: The tosylate anion is a weak base, meaning it doesn't readily accept a proton. This characteristic contributes to its ease of departure from the substrate.
-
Polarizability: The relatively large size of the tosylate anion contributes to its polarizability, further enhancing its stability as a leaving group.
-
Solubility: OTS derivatives often exhibit good solubility in common organic solvents, making them practical for various synthetic applications.
Comparison with other Leaving Groups:
Let's compare OTS with other common leaving groups to better understand its standing:
| Leaving Group | Basicity | Stability | Polarizability | Size | Notes |
|---|---|---|---|---|---|
| OTS (Tosylate) | Weak | High (Resonance stabilized) | Moderate to High | Moderate | Versatile, widely used |
| Mesylate (Ms) | Weak | High (Resonance stabilized) | Moderate | Smaller than OTS | Similar to OTS but less sterically hindered |
| Triflate (OTf) | Weak | Very High (Resonance stabilized) | High | Moderate | Excellent leaving group, very reactive |
| Iodide (I⁻) | Very Weak | High | High | Large | Excellent leaving group, readily available |
| Bromide (Br⁻) | Weak | Moderate | Moderate | Moderate | Good leaving group, less reactive than iodide |
| Chloride (Cl⁻) | Weak | Moderate | Moderate | Small | Fair leaving group, less reactive than bromide |
| Hydroxide (OH⁻) | Strong | Low | Low | Small | Poor leaving group; often needs activation |
| Acetate (OAc⁻) | Weak | Moderate | Moderate | Small | Moderate leaving group |
As the table shows, OTS falls into the category of excellent leaving groups, comparable to mesylate and even surpassing some others in certain reactions due to its specific properties. Triflate is arguably a superior leaving group due to its even greater resonance stabilization, but OTS offers a good balance of reactivity and ease of synthesis.
Applications of OTS in Organic Synthesis
OTS finds extensive use in various organic reactions, including:
-
SN1 and SN2 Reactions: OTS is a versatile leaving group in both SN1 (substitution nucleophilic unimolecular) and SN2 (substitution nucleophilic bimolecular) reactions. Its ability to stabilize the developing negative charge makes it suitable for both mechanisms.
-
Elimination Reactions (E1 and E2): OTS can also participate in elimination reactions, where a leaving group and a proton are removed from adjacent carbon atoms to form a double bond. Again, its stability facilitates the process.
-
Esterification Reactions: While primarily known as a leaving group, the tosylate group itself can participate in esterification reactions.
-
Protecting Groups: Although not its primary function, OTS can serve as a protecting group for alcohols in certain synthetic strategies. This protection prevents unwanted reactions on the alcohol functionality while other transformations are performed on the molecule.
-
Synthesis of Ethers and other Functional Groups: OTS is frequently employed in the synthesis of ethers through Williamson ether synthesis, where an alkoxide reacts with an alkyl tosylate to form an ether linkage. Similar strategies can be applied to synthesize other functional groups.
Factors Affecting OTS's Leaving Group Ability
While OTS is generally a good leaving group, several factors can influence its effectiveness:
-
Steric Hindrance: Bulky groups around the carbon atom bearing the OTS group can hinder the approach of nucleophiles in SN2 reactions.
-
Solvent Effects: The solvent used can significantly impact the reaction rate. Polar aprotic solvents typically favor SN2 reactions, enhancing the effectiveness of OTS as a leaving group in such cases.
-
Nature of the Nucleophile: A stronger nucleophile will generally react faster with an alkyl tosylate, regardless of the specific reaction mechanism.
-
Temperature: Higher temperatures can accelerate reactions involving OTS as a leaving group, increasing the reaction rate.
Conclusion: OTS – A Valuable Tool in the Synthetic Chemist's Arsenal
In conclusion, OTS is indeed a good leaving group, possessing several characteristics that make it highly effective in a range of organic reactions. Its resonance-stabilized structure, weak basicity, and moderate size contribute to its efficacy. While it may not always be the absolute best leaving group in every scenario (triflate frequently holds that title), its versatility, readily available synthesis, and ease of use make it a valuable and frequently employed reagent in organic synthesis. The choice of leaving group often depends on the specific reaction and desired outcome, but OTS remains a reliable and powerful tool for synthetic chemists. Understanding its properties and limitations is essential for successfully planning and executing various organic transformations. Further research into its applications in novel synthetic methods continues to expand its importance in modern organic chemistry.
Latest Posts
Latest Posts
-
What Is Fusion In Chemistry Phase Changes
Mar 24, 2025
-
Does Gas Have A Fixed Volume
Mar 24, 2025
-
A Compound Held Together By Ionic Bonds Is Called
Mar 24, 2025
-
Interval Of Convergence Of Taylor Series
Mar 24, 2025
-
Do Diastereomers Have The Same Physical Properties
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about Is Ots A Good Leaving Group . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
