Secondary Structure Of Proteins Are Stabilized By
Muz Play
Mar 26, 2025 · 6 min read
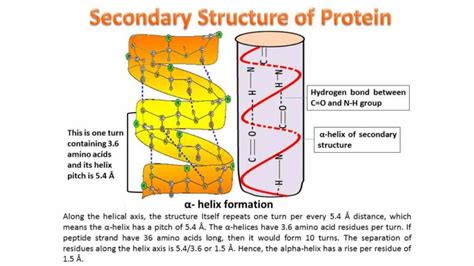
Table of Contents
Secondary Structure of Proteins: Stabilized by Hydrogen Bonds and More
The three-dimensional structure of a protein is crucial to its function. This structure is not a random arrangement of amino acids but rather a highly organized hierarchy. The first level of this organization is the primary structure, simply the linear sequence of amino acids. Building upon this foundation, the protein folds into secondary structures, stabilized primarily by hydrogen bonds. However, other factors play significant, albeit often lesser, roles in stabilizing these essential structural elements. This article will delve deep into the intricacies of protein secondary structure stabilization, exploring the dominant role of hydrogen bonding and the contributing effects of other forces.
The Cornerstones of Secondary Structure: α-Helices and β-Sheets
The most common secondary structures are the α-helix and the β-sheet. Both structures arise from the backbone interactions of the polypeptide chain, specifically hydrogen bonding between the carbonyl oxygen of one amino acid and the amide hydrogen of another. Let's examine each in detail:
The α-Helix: A Tight Spiral
The α-helix is a right-handed coil, resembling a spiral staircase. The hydrogen bonds in an α-helix are formed between the carbonyl oxygen of the nth amino acid residue and the amide hydrogen of the *(n+4)*th residue. This regular pattern creates a stable, repeating structure. Each turn of the helix encompasses approximately 3.6 amino acid residues, with a rise of 5.4 Å per turn.
Factors Influencing α-Helix Stability:
-
Hydrogen Bonding: The backbone hydrogen bonds are the primary stabilizing force. The strength and number of these bonds are crucial. Proline and glycine, due to their unique structures, are helix breakers. Proline's rigid cyclic structure disrupts the regular helix conformation, while glycine's flexibility allows for alternative conformations, reducing the stability of the α-helix.
-
Steric Hindrance: Bulky side chains can clash, hindering helix formation. The presence of multiple large, branched amino acids close together can destabilize the helix.
-
Electrostatic Interactions: Repulsive interactions between charged amino acid side chains can destabilize the α-helix. Similarly, attractive interactions between oppositely charged residues can stabilize it.
-
Solvent Effects: The surrounding environment, including the solvent (e.g., water), can influence the stability of the helix. Hydrophobic interactions between nonpolar side chains can favor helix formation in hydrophobic environments.
β-Sheets: Extended Structures
β-sheets are formed by extended polypeptide chains arranged side-by-side. These strands are linked by hydrogen bonds between adjacent strands. The hydrogen bonds are formed between the carbonyl oxygen of one strand and the amide hydrogen of another. β-sheets can be either parallel (strands running in the same direction) or antiparallel (strands running in opposite directions). Antiparallel β-sheets are generally more stable due to the near linearity of the hydrogen bonds.
Factors Influencing β-Sheet Stability:
-
Hydrogen Bonding: Similar to α-helices, hydrogen bonding is the primary force stabilizing β-sheets. The strength and number of these bonds are critical for stability.
-
Side Chain Interactions: The side chains of amino acids in adjacent strands can interact with each other. These interactions, which can be hydrophobic or electrostatic, can contribute to the overall stability of the sheet.
-
Steric Hindrance: Similar to α-helices, steric clashes between bulky side chains can destabilize the β-sheet.
-
Twisting: β-sheets often exhibit a slight twist, minimizing steric clashes and enhancing stability.
Beyond Hydrogen Bonds: Other Stabilizing Forces
While hydrogen bonds are the dominant force, other interactions play supporting roles in stabilizing secondary structures:
1. Hydrophobic Interactions: The Power of Avoiding Water
Hydrophobic amino acid side chains tend to cluster together in the protein's interior, minimizing their contact with water. This hydrophobic effect is a powerful driving force in protein folding, indirectly influencing the formation and stability of secondary structures. Hydrophobic interactions contribute to the stability of both α-helices and β-sheets by promoting the proper arrangement of hydrophobic residues within the protein core.
2. Electrostatic Interactions: Charges Matter
Electrostatic interactions between charged amino acid side chains can influence the stability of secondary structures. Attractive interactions between oppositely charged residues stabilize the structure, while repulsive interactions between like charges destabilize it. These interactions are particularly important in regions where charged residues are clustered close together. Salt bridges, a specific type of strong electrostatic interaction between oppositely charged amino acids, can significantly contribute to the overall stability.
3. Van der Waals Forces: Weak but Numerous
Van der Waals forces are weak, short-range attractions between atoms. While individually weak, the cumulative effect of numerous van der Waals interactions throughout the secondary structure can contribute significantly to its overall stability. These forces are particularly important in densely packed regions of the protein.
4. Peptide Bond Planarity: Structural Constraints
The peptide bond, a crucial component of the polypeptide backbone, exhibits partial double bond character. This limits rotation around the peptide bond, imposing constraints on the conformation of the polypeptide chain and influencing the formation of secondary structures. The planarity of the peptide bond is essential for the precise geometry required for effective hydrogen bonding in both α-helices and β-sheets.
The Importance of Context: Factors Affecting Stability in the Entire Protein
It's crucial to remember that the stability of secondary structures isn't solely determined by local interactions. The overall context within the protein plays a significant role:
-
Tertiary Structure Interactions: The interactions between different secondary structural elements, forming the tertiary structure, significantly influence the stability of individual secondary structures. Packing of secondary structures against each other and the overall three-dimensional arrangement of the protein profoundly impact their stability. For example, a buried α-helix might be more stable than an exposed one due to favorable hydrophobic interactions.
-
Quaternary Structure (for multimeric proteins): In proteins composed of multiple subunits (quaternary structure), interactions between subunits can also affect secondary structure stability. Interactions between subunits can help stabilize secondary structural elements that might otherwise be less stable in isolation.
Techniques for Studying Secondary Structure
Various experimental techniques can be used to determine the secondary structure content of a protein:
-
Circular Dichroism (CD) Spectroscopy: CD spectroscopy measures the differential absorption of left and right circularly polarized light. The resulting CD spectrum provides information about the secondary structure content of the protein.
-
Nuclear Magnetic Resonance (NMR) Spectroscopy: NMR spectroscopy provides detailed information about the three-dimensional structure of proteins, including secondary structure elements.
-
X-ray Crystallography: X-ray crystallography allows for high-resolution determination of protein structures, including accurate identification and characterization of secondary structure elements.
-
Infrared (IR) Spectroscopy: IR spectroscopy can identify specific vibrational modes associated with secondary structure elements, enabling the determination of secondary structure content.
Conclusion: A Collaborative Effort
The stability of protein secondary structures is a consequence of a complex interplay between various forces. While hydrogen bonds are undeniably the primary driving force, hydrophobic interactions, electrostatic interactions, van der Waals forces, and the inherent planarity of the peptide bond all contribute significantly to their overall stability. The specific contribution of each force depends on the amino acid sequence, the specific type of secondary structure (α-helix or β-sheet), and the overall context within the protein's three-dimensional structure. Understanding these stabilizing forces is fundamental to understanding protein structure and function and continues to be an active area of research. The ongoing advancements in experimental techniques and computational modeling are constantly refining our understanding of this intricate and vital aspect of biological molecules.
Latest Posts
Latest Posts
-
What Is Stronger C C Bond Or C Cl Bond
Mar 29, 2025
-
Where Is The Bacterial Chromosome Located
Mar 29, 2025
-
Write The Chemical Formula For This Molecule
Mar 29, 2025
-
How To Calculate Velocity From Flow Rate
Mar 29, 2025
-
Write The Iupac Names Of The Given Carboxylic Acids
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Secondary Structure Of Proteins Are Stabilized By . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
