Sodium Acetate And Acetic Acid Buffer
Muz Play
Mar 26, 2025 · 6 min read
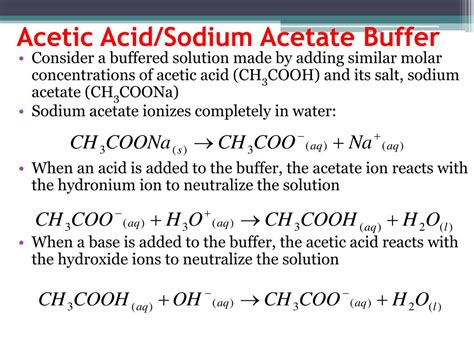
Table of Contents
Sodium Acetate and Acetic Acid Buffer: A Comprehensive Guide
The sodium acetate and acetic acid buffer system is a classic example of a weak acid-conjugate base buffer. Understanding its properties, preparation, and applications is crucial in various fields, from chemistry and biology to medicine and environmental science. This comprehensive guide will delve into the intricacies of this buffer system, exploring its mechanism, applications, limitations, and practical considerations.
Understanding Buffer Solutions
Before diving into the specifics of the sodium acetate/acetic acid buffer, let's establish a fundamental understanding of buffer solutions themselves. A buffer solution, also known as a buffer, is an aqueous solution that resists changes in pH upon the addition of small amounts of acid or base. This resistance to pH change is crucial in many biological and chemical processes where maintaining a stable pH is essential.
Buffers achieve this stability through the presence of a weak acid and its conjugate base (or a weak base and its conjugate acid). The equilibrium between the weak acid and its conjugate base allows the buffer to neutralize added H⁺ or OH⁻ ions, minimizing the impact on the overall pH.
The Sodium Acetate/Acetic Acid Buffer System
The sodium acetate/acetic acid buffer system utilizes acetic acid (CH₃COOH), a weak acid, and its conjugate base, sodium acetate (CH₃COONa). Acetic acid partially dissociates in water, establishing an equilibrium:
CH₃COOH ⇌ CH₃COO⁻ + H⁺
The addition of sodium acetate provides a significant concentration of acetate ions (CH₃COO⁻), shifting the equilibrium to the left. This presence of both the weak acid (CH₃COOH) and its conjugate base (CH₃COO⁻) allows the buffer to effectively resist pH changes.
How it Works: The Buffering Mechanism
The effectiveness of the buffer stems from its ability to neutralize both added acids and bases.
-
Addition of a strong acid (e.g., HCl): The added H⁺ ions react with the acetate ions (CH₃COO⁻) to form acetic acid (CH₃COOH):
H⁺ + CH₃COO⁻ ⇌ CH₃COOH
This reaction consumes the added H⁺ ions, preventing a significant decrease in pH.
-
Addition of a strong base (e.g., NaOH): The added OH⁻ ions react with the acetic acid (CH₃COOH) to form water and acetate ions (CH₃COO⁻):
OH⁻ + CH₃COOH ⇌ CH₃COO⁻ + H₂O
This reaction consumes the added OH⁻ ions, preventing a significant increase in pH.
Calculating the pH of a Sodium Acetate/Acetic Acid Buffer
The pH of a sodium acetate/acetic acid buffer can be calculated using the Henderson-Hasselbalch equation:
pH = pKa + log([CH₃COO⁻]/[CH₃COOH])
Where:
- pH is the pH of the buffer solution.
- pKa is the negative logarithm of the acid dissociation constant (Ka) of acetic acid (approximately 4.76 at 25°C).
- [CH₃COO⁻] is the concentration of acetate ions (from sodium acetate).
- [CH₃COOH] is the concentration of acetic acid.
This equation highlights the importance of the ratio of conjugate base to weak acid in determining the buffer's pH. A ratio of 1:1 results in a pH equal to the pKa. Adjusting this ratio allows for fine-tuning the buffer's pH to the desired value.
Preparing a Sodium Acetate/Acetic Acid Buffer
Preparing a sodium acetate/acetic acid buffer typically involves dissolving appropriate amounts of acetic acid and sodium acetate in water. The exact quantities depend on the desired buffer concentration and pH. Here's a general outline:
-
Calculate the required amounts: Use the Henderson-Hasselbalch equation to determine the necessary concentrations of acetic acid and sodium acetate to achieve the target pH.
-
Dissolve the components: Carefully weigh out the calculated amounts of acetic acid and sodium acetate. Dissolve them separately in a portion of the total desired volume of water.
-
Combine the solutions: Combine the acetic acid and sodium acetate solutions. Add more water to bring the solution to the final desired volume.
-
Measure the pH: Use a pH meter to verify the buffer's pH. Adjust the pH if necessary by adding small amounts of acid or base.
Applications of Sodium Acetate/Acetic Acid Buffer
The sodium acetate/acetic acid buffer finds widespread applications across various scientific and industrial fields due to its relatively non-toxic nature and its buffering capacity within a physiological pH range. Some notable applications include:
-
Biological research: Maintaining a stable pH is crucial in numerous biological experiments, cell cultures, and enzyme assays. The sodium acetate/acetic acid buffer is frequently employed in these applications due to its compatibility with biological systems.
-
Food preservation: Acetic acid, a key component of the buffer, acts as a natural preservative, inhibiting bacterial growth. The buffer system can help maintain a controlled pH environment in food products.
-
Textile industry: The buffer can be used to control pH during dyeing and finishing processes in textile manufacturing. Controlling pH is essential to maintain the quality and color of fabrics.
-
Pharmaceutical industry: The buffer system can be used in the formulation of certain pharmaceuticals where pH control is critical for stability and effectiveness.
-
Analytical chemistry: Buffers are frequently used in titrations and other analytical procedures to maintain a stable pH environment, leading to more accurate and reliable results.
Limitations of Sodium Acetate/Acetic Acid Buffer
While the sodium acetate/acetic acid buffer offers numerous advantages, it also possesses limitations:
-
Limited buffering capacity: The buffer's capacity is limited by the concentrations of acetic acid and sodium acetate. Adding excessive amounts of acid or base will eventually overwhelm the buffer, causing significant pH changes.
-
Temperature dependence: The pKa of acetic acid is temperature-dependent. Therefore, the buffer's pH will also vary with temperature changes. This can be significant for applications where temperature fluctuations are considerable.
-
Ionic strength effects: The ionic strength of the buffer solution can affect its pH. This is important to consider when dealing with solutions containing high concentrations of other ions.
-
Not suitable for extreme pH ranges: The sodium acetate/acetic acid buffer is most effective within a pH range of approximately 3.76 to 5.76 (pKa ± 1). It is not suitable for applications requiring buffer solutions at extreme pH values.
Choosing the Right Buffer
Selecting the appropriate buffer for a particular application requires careful consideration of several factors:
-
Desired pH range: The buffer's pKa should be close to the target pH to maximize its buffering capacity.
-
Buffering capacity: The buffer's capacity should be sufficient to withstand the anticipated addition of acid or base.
-
Solubility: The buffer components should be readily soluble in the solvent used.
-
Toxicity: The buffer components should be non-toxic or minimally toxic, particularly for biological applications.
-
Ionic strength: The buffer's ionic strength should be compatible with other components in the system.
Conclusion
The sodium acetate/acetic acid buffer is a versatile and widely used system for controlling pH in a variety of applications. Its simplicity, relatively low cost, and non-toxicity make it a popular choice in many fields. However, it is essential to understand its limitations and carefully consider the specific requirements of the application before selecting this or any other buffer system. By understanding the principles behind buffer solutions and the specific characteristics of the sodium acetate/acetic acid buffer, researchers and practitioners can effectively utilize this tool to maintain stable pH environments in various chemical and biological processes. Further research into more specialized buffer systems may be necessary for applications requiring highly specific pH control or extreme pH ranges.
Latest Posts
Latest Posts
-
In A Chemical Reaction What Are The Reactants And Products
Mar 29, 2025
-
What Elements Are Gases At Room Temperature
Mar 29, 2025
-
Why Is Funding For Schools From Sponsorships A Bad Thing
Mar 29, 2025
-
Mg Oh 2 Is Acid Or Base
Mar 29, 2025
-
What Is The Density Of Maple Syrup
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Sodium Acetate And Acetic Acid Buffer . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
